[English] 日本語
 Yorodumi
Yorodumi- EMDB-22370: Cryo-EM structure of MDA5-dsRNA filament in complex with TRIM65 P... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22370 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of MDA5-dsRNA filament in complex with TRIM65 PSpry domain (Trimer) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA helicase / Ubiquitin E3 ligase / innate immunity / dsRNA / TRIM family / ATPase / HYDROLASE-IMMUNE SYSTEM-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of protein oligomerization / MDA-5 signaling pathway / regulation of type III interferon production / detection of virus / negative regulation of NLRP3 inflammasome complex assembly / positive regulation of response to cytokine stimulus / NF-kB activation through FADD/RIP-1 pathway mediated by caspase-8 and -10 / Modulation of host responses by IFN-stimulated genes / TRAF6 mediated IRF7 activation / negative regulation of viral genome replication ...positive regulation of protein oligomerization / MDA-5 signaling pathway / regulation of type III interferon production / detection of virus / negative regulation of NLRP3 inflammasome complex assembly / positive regulation of response to cytokine stimulus / NF-kB activation through FADD/RIP-1 pathway mediated by caspase-8 and -10 / Modulation of host responses by IFN-stimulated genes / TRAF6 mediated IRF7 activation / negative regulation of viral genome replication / type I interferon-mediated signaling pathway / cytoplasmic pattern recognition receptor signaling pathway / cellular response to exogenous dsRNA / pattern recognition receptor activity / TRAF6 mediated NF-kB activation / protein complex oligomerization / positive regulation of interferon-alpha production / protein sumoylation / protein K63-linked ubiquitination / ribonucleoprotein complex binding / protein K48-linked ubiquitination / positive regulation of autophagy / antiviral innate immune response / positive regulation of interferon-beta production / Negative regulators of DDX58/IFIH1 signaling / DDX58/IFIH1-mediated induction of interferon-alpha/beta / cellular response to virus / RING-type E3 ubiquitin transferase / Evasion by RSV of host interferon responses / positive regulation of interleukin-6 production / negative regulation of inflammatory response / response to virus / positive regulation of tumor necrosis factor production / SARS-CoV-1 activates/modulates innate immune responses / ubiquitin-protein transferase activity / Ovarian tumor domain proteases / ubiquitin protein ligase activity / double-stranded RNA binding / TRAF3-dependent IRF activation pathway / defense response to virus / RNA helicase activity / Ub-specific processing proteases / single-stranded RNA binding / RNA helicase / protein domain specific binding / innate immune response / SARS-CoV-2 activates/modulates innate and adaptive immune responses / ATP hydrolysis activity / mitochondrion / DNA binding / RNA binding / zinc ion binding / nucleoplasm / ATP binding / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Kato K / Ahmad S | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2021 Journal: Mol Cell / Year: 2021Title: Structural analysis of RIG-I-like receptors reveals ancient rules of engagement between diverse RNA helicases and TRIM ubiquitin ligases. Authors: Kazuki Kato / Sadeem Ahmad / Zixiang Zhu / Janet M Young / Xin Mu / Sehoon Park / Harmit S Malik / Sun Hur /   Abstract: RNA helicases and E3 ubiquitin ligases mediate many critical functions in cells, but their actions have largely been studied in distinct biological contexts. Here, we uncover evolutionarily conserved ...RNA helicases and E3 ubiquitin ligases mediate many critical functions in cells, but their actions have largely been studied in distinct biological contexts. Here, we uncover evolutionarily conserved rules of engagement between RNA helicases and tripartite motif (TRIM) E3 ligases that lead to their functional coordination in vertebrate innate immunity. Using cryoelectron microscopy and biochemistry, we show that RIG-I-like receptors (RLRs), viral RNA receptors with helicase domains, interact with their cognate TRIM/TRIM-like E3 ligases through similar epitopes in the helicase domains. Their interactions are avidity driven, restricting the actions of TRIM/TRIM-like proteins and consequent immune activation to RLR multimers. Mass spectrometry and phylogeny-guided biochemical analyses further reveal that similar rules of engagement may apply to diverse RNA helicases and TRIM/TRIM-like proteins. Our analyses suggest not only conserved substrates for TRIM proteins but also, unexpectedly, deep evolutionary connections between TRIM proteins and RNA helicases, linking ubiquitin and RNA biology throughout animal evolution. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22370.map.gz emd_22370.map.gz | 8.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22370-v30.xml emd-22370-v30.xml emd-22370.xml emd-22370.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22370.png emd_22370.png | 37 KB | ||
| Filedesc metadata |  emd-22370.cif.gz emd-22370.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22370 http://ftp.pdbj.org/pub/emdb/structures/EMD-22370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22370 | HTTPS FTP |
-Related structure data
| Related structure data |  7jl2MC  7jl0C  7jl1C  7jl3C  7jl4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22370.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22370.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03594 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
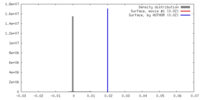
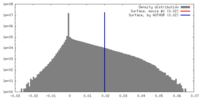
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ternary complex of MDA5-dsRNA-TRIM65
| Entire | Name: Ternary complex of MDA5-dsRNA-TRIM65 |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of MDA5-dsRNA-TRIM65
| Supramolecule | Name: Ternary complex of MDA5-dsRNA-TRIM65 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: RNA (44-MER)
| Macromolecule | Name: RNA (44-MER) / type: rna / ID: 1 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.208519 KDa |
| Sequence | String: GACUGACUGA CUGAAGACUG ACUGACUGAA GACUGACUGA CUGA |
-Macromolecule #2: RNA (44-MER)
| Macromolecule | Name: RNA (44-MER) / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.973247 KDa |
| Sequence | String: UCAGUCAGUC AGUCUUCAGU CAGUCAGUCU UCAGUCAGUC AGUC |
-Macromolecule #3: Interferon-induced helicase C domain-containing protein 1
| Macromolecule | Name: Interferon-induced helicase C domain-containing protein 1 type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO / EC number: RNA helicase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 84.901695 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSDSDEENV AARASPEPEL QLRPYQMEVA QPALEGKNII ICLPTGSGKT RVAVYIAKDH LDKKKKASEP GKVIVLVNKV LLVEQLFRK EFQPFLKKWY RVIGLSGDTQ LKISFPEVVK SCDIIISTAQ ILENSLLNLE NGEDAGVQLS DFSLIIIDEC H HTNKEAVY ...String: MGSDSDEENV AARASPEPEL QLRPYQMEVA QPALEGKNII ICLPTGSGKT RVAVYIAKDH LDKKKKASEP GKVIVLVNKV LLVEQLFRK EFQPFLKKWY RVIGLSGDTQ LKISFPEVVK SCDIIISTAQ ILENSLLNLE NGEDAGVQLS DFSLIIIDEC H HTNKEAVY NNIMRHYLMQ KLKNNRLKKE NKPVIPLPQI LGLTASPGVG GATKQAKAEE HILKLCANLD AFTIKTVKEN LD QLKNQIQ EPCKKFAIAD ATREDPFKEK LLEIMTRIQT YCQMSPMSDF GTQPYEQWAI QMEKKAAKEG NRKERVCAEH LRK YNEALQ INDTIRMIDA YTHLETFYNE EKDKKFAVIE DDSDEGGDDE YCDGDEDEDD LKKPLKLDET DRFLMTLFFE NNKM LKRLA ENPEYENEKL TKLRNTIMEQ YTRTEESARG IIFTKTRQSA YALSQWITEN EKFAEVGVKA HHLIGAGHSS EFKPM TQNE QKEVISKFRT GKINLLIATT VAEEGLDIKE CNIVIRYGLV TNEIAMVQAR GRARADESTY VLVAHSGSGV IERETV NDF REKMMYKAIH CVQNMKPEEY AHKILELQMQ SIMEKKMKTK RNIAKHYKNN PSLITFLCKN CSVLACSGED IHVIEKM HH VNMTPEFKEL YIVREKKTLQ KKCADYQING EIICKCGQAW GTMMVHKGLD LPCLKIRNFV VVFKNNSTKK QYKKWVEL P ITFPNLDYSE CCLFSDED UniProtKB: Interferon-induced helicase C domain-containing protein 1 |
-Macromolecule #4: Tripartite motif-containing protein 65
| Macromolecule | Name: Tripartite motif-containing protein 65 / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.643365 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LAPVPSTVCP LRRKLWQNYR NLTFDPVSAN RHFYLSRQDQ QVKHLRQSRG PGGPGSFELW QVQCAQSFQA GHHYWEVRAS DHSVTLGVS YPQLPRSRLG PHTDNIGRGP SSWGLCVQED SLQAWHNGEA QRLPGVSGRL LGMDLDLASG CLTFYSLEPQ T QPLYTFHA LFNQPLTPVF WLLEGRTLTL CHQ UniProtKB: E3 ubiquitin-protein ligase TRIM65 |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 3 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 3 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #7: TETRAFLUOROALUMINATE ION
| Macromolecule | Name: TETRAFLUOROALUMINATE ION / type: ligand / ID: 7 / Number of copies: 3 / Formula: ALF |
|---|---|
| Molecular weight | Theoretical: 102.975 Da |
| Chemical component information |  ChemComp-ALF: |
-Macromolecule #8: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 8 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 72.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 46.8426 Å Applied symmetry - Helical parameters - Δ&Phi: 86.9406 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 15306 |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Startup model | Type of model: OTHER / Details: Featureless cylinder |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)