[English] 日本語
 Yorodumi
Yorodumi- EMDB-22212: Structure of the human mitochondrial ribosome-EF-G1 complex (ClassII) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22212 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human mitochondrial ribosome-EF-G1 complex (ClassII) | |||||||||
 Map data Map data | ClassII 55S-EF-G1mt complex | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.96 Å | |||||||||
 Authors Authors | Sharma MR / Koripella RK / Agrawal RK | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation. Authors: Ravi Kiran Koripella / Manjuli R Sharma / Kalpana Bhargava / Partha P Datta / Prem S Kaushal / Pooja Keshavan / Linda L Spremulli / Nilesh K Banavali / Rajendra K Agrawal /   Abstract: The mammalian mitochondrial ribosome (mitoribosome) and its associated translational factors have evolved to accommodate greater participation of proteins in mitochondrial translation. Here we ...The mammalian mitochondrial ribosome (mitoribosome) and its associated translational factors have evolved to accommodate greater participation of proteins in mitochondrial translation. Here we present the 2.68-3.96 Å cryo-EM structures of the human 55S mitoribosome in complex with the human mitochondrial elongation factor G1 (EF-G1) in three distinct conformational states, including an intermediate state and a post-translocational state. These structures reveal the role of several mitochondria-specific (mito-specific) mitoribosomal proteins (MRPs) and a mito-specific segment of EF-G1 in mitochondrial tRNA (tRNA) translocation. In particular, the mito-specific C-terminal extension in EF-G1 is directly involved in translocation of the acceptor arm of the A-site tRNA. In addition to the ratchet-like and independent head-swiveling motions exhibited by the small mitoribosomal subunit, we discover significant conformational changes in MRP mL45 at the nascent polypeptide-exit site within the large mitoribosomal subunit that could be critical for tethering of the elongating mitoribosome onto the inner-mitochondrial membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22212.map.gz emd_22212.map.gz | 224.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22212-v30.xml emd-22212-v30.xml emd-22212.xml emd-22212.xml | 8.7 KB 8.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22212.png emd_22212.png | 219.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22212 http://ftp.pdbj.org/pub/emdb/structures/EMD-22212 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22212 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22212 | HTTPS FTP |
-Related structure data
| Related structure data |  6vlzC  6vmiC C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10481 (Title: Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation EMPIAR-10481 (Title: Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongationData size: 753.5 Data #1: Aligned single-frame particles of human mitochondrial 55S-EF-Gmt complex [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22212.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22212.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ClassII 55S-EF-G1mt complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0961 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
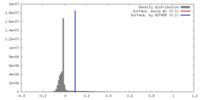
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human mitochondrial ribosome-EF-G1 complex
| Entire | Name: Human mitochondrial ribosome-EF-G1 complex |
|---|---|
| Components |
|
-Supramolecule #1: Human mitochondrial ribosome-EF-G1 complex
| Supramolecule | Name: Human mitochondrial ribosome-EF-G1 complex / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | #0 - Image recording ID: 1 / #0 - Film or detector model: GATAN K2 SUMMIT (4k x 4k) / #0 - Average electron dose: 69.21 e/Å2 / #1 - Image recording ID: 2 #1 - Film or detector model: DIRECT ELECTRON DE-16 (4k x 4k) #1 - Detector mode: COUNTING / #1 - Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: OTHER |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)