+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22182 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | YSD1 bacteriophage capsid | ||||||||||||
 Map data Map data | Sharpened masked map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Bacteriophage capsid / VIRUS | ||||||||||||
| Function / homology | Head decoration protein D / Bacteriophage lambda head decoration protein D / Major capsid protein GpE / Phage major capsid protein E / viral capsid / host cell cytoplasm / Putative+head+decorative+protein / Major capsid protein Function and homology information Function and homology information | ||||||||||||
| Biological species |  Bacteriophage sp. (virus) Bacteriophage sp. (virus) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Hardy JM / Dunstan R | ||||||||||||
| Funding support |  Australia, Australia,  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: The architecture and stabilisation of flagellotropic tailed bacteriophages. Authors: Joshua M Hardy / Rhys A Dunstan / Rhys Grinter / Matthew J Belousoff / Jiawei Wang / Derek Pickard / Hariprasad Venugopal / Gordon Dougan / Trevor Lithgow / Fasséli Coulibaly /   Abstract: Flagellotropic bacteriophages engage flagella to reach the bacterial surface as an effective means to increase the capture radius for predation. Structural details of these viruses are of great ...Flagellotropic bacteriophages engage flagella to reach the bacterial surface as an effective means to increase the capture radius for predation. Structural details of these viruses are of great interest given the substantial drag forces and torques they face when moving down the spinning flagellum. We show that the main capsid and auxiliary proteins form two nested chainmails that ensure the integrity of the bacteriophage head. Core stabilising structures are conserved in herpesviruses suggesting their ancestral origin. The structure of the tail also reveals a robust yet pliable assembly. Hexameric rings of the tail-tube protein are braced by the N-terminus and a β-hairpin loop, and interconnected along the tail by the splayed β-hairpins. By contrast, we show that the β-hairpin has an inhibitory role in the tail-tube precursor, preventing uncontrolled self-assembly. Dyads of acidic residues inside the tail-tube present regularly-spaced motifs well suited to DNA translocation into bacteria through the tail. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22182.map.gz emd_22182.map.gz | 195.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22182-v30.xml emd-22182-v30.xml emd-22182.xml emd-22182.xml | 22.3 KB 22.3 KB | Display Display |  EMDB header EMDB header |
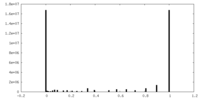
| FSC (resolution estimation) |  emd_22182_fsc.xml emd_22182_fsc.xml | 28.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_22182.png emd_22182.png | 262 KB | ||
| Masks |  emd_22182_msk_1.map emd_22182_msk_1.map | 824 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22182.cif.gz emd-22182.cif.gz | 6.4 KB | ||
| Others |  emd_22182_additional.map.gz emd_22182_additional.map.gz emd_22182_half_map_1.map.gz emd_22182_half_map_1.map.gz emd_22182_half_map_2.map.gz emd_22182_half_map_2.map.gz | 763.7 MB 748.3 MB 749.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22182 http://ftp.pdbj.org/pub/emdb/structures/EMD-22182 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22182 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22182 | HTTPS FTP |
-Related structure data
| Related structure data |  6xgqMC  6xgpC  6xgrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22182.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22182.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened masked map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
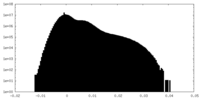
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22182_msk_1.map emd_22182_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map
| File | emd_22182_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_22182_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_22182_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bacteriophage sp.
| Entire | Name:  Bacteriophage sp. (virus) Bacteriophage sp. (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Bacteriophage sp.
| Supramolecule | Name: Bacteriophage sp. / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: From environmental water samples taken during a phage survey of the waterways of Cambridge UK, the phage YSD1 was isolated using the attenuated S. enterica serovar Typhi BRD948. The virus ...Details: From environmental water samples taken during a phage survey of the waterways of Cambridge UK, the phage YSD1 was isolated using the attenuated S. enterica serovar Typhi BRD948. The virus was then amplified by infecting S. Typhimurium SL3261 delta-fljB. NCBI-ID: 38018 / Sci species name: Bacteriophage sp. / Sci species strain: YSD1 / Virus type: VIRION / Virus isolate: SUBSPECIES / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhi (bacteria) Salmonella enterica subsp. enterica serovar Typhi (bacteria) |
| Molecular weight | Theoretical: 22.8 MDa |
| Virus shell | Shell ID: 1 / Name: YSD1 capsid / Diameter: 650.0 Å / T number (triangulation number): 7 |
-Macromolecule #1: YSD1_17
| Macromolecule | Name: YSD1_17 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteriophage sp. (virus) Bacteriophage sp. (virus) |
| Molecular weight | Theoretical: 39.945598 KDa |
| Sequence | String: MAGLYTTYQL LEVQRKLKTL PAFFLQWFPR QINFQEDMIA FDKVIQDVTR VAPFVAPNVQ GRVIKESGYN TKTFKPAYVK PKHVIDPNM IIPRQPGEAL GTGTLSIAQR RDRVIAYLLM KHRAMHENTW EWMAAQAAQY GYVDVQGQDY PLVRVDFGRD A ALTMTTDW ...String: MAGLYTTYQL LEVQRKLKTL PAFFLQWFPR QINFQEDMIA FDKVIQDVTR VAPFVAPNVQ GRVIKESGYN TKTFKPAYVK PKHVIDPNM IIPRQPGEAL GTGTLSIAQR RDRVIAYLLM KHRAMHENTW EWMAAQAAQY GYVDVQGQDY PLVRVDFGRD A ALTMTTDW TAAGVTLMDM IADLRDGQRL VSDKSMSGTV IRDYVFGGDA WDQFVKVGGK ELWGKDGLMD STIRGSETNV TR LWDDVEG VQYMGELVGA NGAGRMRIWV NTQKYRDQND QEQFLMKQKA VMGISSAIEG VRCFGAILDK GAGYQALDYF PKM WDQEDP SVEYLMSQGA PLMVPADPNA SFLLTVMS UniProtKB: Major capsid protein |
-Macromolecule #2: YSD1_16
| Macromolecule | Name: YSD1_16 / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteriophage sp. (virus) Bacteriophage sp. (virus) |
| Molecular weight | Theoretical: 14.401047 KDa |
| Sequence | String: MNLLTMMAAT SLPNYLAGNG DLGSWEPTQI FAGEADIVTE GGAAGADIEI YQVIAKNAAG AMVPHDPTAT TGTSPDEVPA PQSVAIGIA AQPAKSGQNV PYYIGGVFNH AALGWHASLD TLAKRQAVFD RTNIHIGNLY UniProtKB: Putative+head+decorative+protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: The grid was blotted for 2 seconds with a blot force of -3 and no drain time.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-30 / Number grids imaged: 1 / Number real images: 1881 / Average exposure time: 12.0 sec. / Average electron dose: 27.24 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.5 µm / Calibrated defocus min: 0.5 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)