[English] 日本語
 Yorodumi
Yorodumi- EMDB-22155: CryoEM structure of apo G protein-gated inwardly rectifying potas... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22155 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of apo G protein-gated inwardly rectifying potassium channel GIRK2 | |||||||||
 Map data Map data | CryoEM structure of apo GIRK2 | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationG-protein activated inward rectifier potassium channel activity / inward rectifier potassium channel complex Similarity search - Function | |||||||||
| Biological species |  | |||||||||
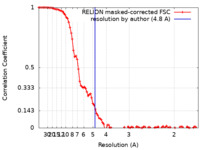
| Method | single particle reconstruction / cryo EM / Resolution: 4.8 Å | |||||||||
 Authors Authors | Mathiharan YK / Glaaser IW / Skiniotis G / Slesinger PA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Structural insights into GIRK2 channel modulation by cholesterol and PIP. Authors: Yamuna Kalyani Mathiharan / Ian W Glaaser / Yulin Zhao / Michael J Robertson / Georgios Skiniotis / Paul A Slesinger /  Abstract: G-protein-gated inwardly rectifying potassium (GIRK) channels are important for determining neuronal excitability. In addition to G proteins, GIRK channels are potentiated by membrane cholesterol, ...G-protein-gated inwardly rectifying potassium (GIRK) channels are important for determining neuronal excitability. In addition to G proteins, GIRK channels are potentiated by membrane cholesterol, which is elevated in the brains of people with neurodegenerative diseases such as Alzheimer's dementia and Parkinson's disease. The structural mechanism of cholesterol modulation of GIRK channels is not well understood. In this study, we present cryo- electron microscopy (cryoEM) structures of GIRK2 in the presence and absence of the cholesterol analog cholesteryl hemisuccinate (CHS) and phosphatidylinositol 4,5-bisphosphate (PIP). The structures reveal that CHS binds near PIP in lipid-facing hydrophobic pockets of the transmembrane domain. Our structural analysis suggests that CHS stabilizes PIP interaction with the channel and promotes engagement of the cytoplasmic domain onto the transmembrane region. Mutagenesis of one of the CHS binding pockets eliminates cholesterol-dependent potentiation of GIRK2. Elucidating the structural mechanisms underlying cholesterol modulation of GIRK2 channels could facilitate the development of therapeutics for treating neurological diseases. VIDEO ABSTRACT. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22155.map.gz emd_22155.map.gz | 58.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22155-v30.xml emd-22155-v30.xml emd-22155.xml emd-22155.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22155_fsc.xml emd_22155_fsc.xml | 9.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_22155.png emd_22155.png | 107.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22155 http://ftp.pdbj.org/pub/emdb/structures/EMD-22155 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22155 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22155 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22155.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22155.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure of apo GIRK2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : G protein-gated inwardly rectifying potassium channel (GIRK2)
| Entire | Name: G protein-gated inwardly rectifying potassium channel (GIRK2) |
|---|---|
| Components |
|
-Supramolecule #1: G protein-gated inwardly rectifying potassium channel (GIRK2)
| Supramolecule | Name: G protein-gated inwardly rectifying potassium channel (GIRK2) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: The cryoEM structure obtained in the absence of modulators cholesteryl hemisuccinate and PIP2. |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
-Macromolecule #1: G protein-gated inwardly rectifying potassium channel (GIRK2)
| Macromolecule | Name: G protein-gated inwardly rectifying potassium channel (GIRK2) type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MAKRKIQRYV RKDGKCNVH H GNVRETYR YL TDIFTTL VDL KWRFNL LIFV MVYTV TWLFF GMIW WLIAYI RGD MDHIEDP SW TPCVTNLN G FVSAFLFSI ETETTIGYGY RVITDKCPE G IILLLIQS VL GSIVNAF MVG CMFVKI SQPK KRAET ...String: MAKRKIQRYV RKDGKCNVH H GNVRETYR YL TDIFTTL VDL KWRFNL LIFV MVYTV TWLFF GMIW WLIAYI RGD MDHIEDP SW TPCVTNLN G FVSAFLFSI ETETTIGYGY RVITDKCPE G IILLLIQS VL GSIVNAF MVG CMFVKI SQPK KRAET LVFST HAVI SMRDGK LCL MFRVGDL RN SHIVEASI R AKLIKSKQT SEGEFIPLNQ TDINVGYYT G DDRLFLVS PL IISHEIN QQS PFWEIS KAQL PKEEL EIVVI LEGM VEATGM TCQ ARSSYIT SE ILWGYRFT P VLTLEDGFY EVDYNSFHET YETSTPSLS A KELAELAN RA ESNSLEV LFQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||||||||
| Details | Apo GIRK2 without the modulators cholesteryl hemisuccinate and PIP2. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 8310 / Average exposure time: 3.0 sec. / Average electron dose: 83.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)