+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-21959 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Colicin E1 fragment in nanodisc-embedded TolC | ||||||||||||
 マップデータ マップデータ | asymmetric map of colicin E1 bound to TolC in nanodisc; cropped, sharpened, and resampled | ||||||||||||
 試料 試料 |
| ||||||||||||
 キーワード キーワード | antibiotic efflux / bacteriocin / TRANSPORT PROTEIN / ANTIMICROBIAL PROTEIN | ||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of ion transmembrane transporter activity / MacAB-TolC complex / pore-forming activity / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / xenobiotic detoxification by transmembrane export across the plasma membrane / bile acid transmembrane transporter activity ...negative regulation of ion transmembrane transporter activity / MacAB-TolC complex / pore-forming activity / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / xenobiotic detoxification by transmembrane export across the plasma membrane / bile acid transmembrane transporter activity / porin activity / bile acid and bile salt transport / monoatomic ion channel activity / efflux transmembrane transporter activity / cell outer membrane / response to toxic substance / outer membrane-bounded periplasmic space / monoatomic ion transmembrane transport / defense response to Gram-negative bacterium / killing of cells of another organism / transmembrane transporter binding / response to xenobiotic stimulus / response to antibiotic / identical protein binding / membrane / plasma membrane 類似検索 - 分子機能 | ||||||||||||
| 生物種 |    | ||||||||||||
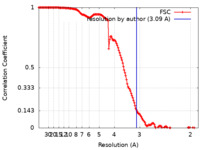
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.09 Å | ||||||||||||
 データ登録者 データ登録者 | Kaelber JT / Budiardjo SJ | ||||||||||||
| 資金援助 |  米国, 3件 米国, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: Elife / 年: 2022 ジャーナル: Elife / 年: 2022タイトル: Colicin E1 opens its hinge to plug TolC. 著者: S Jimmy Budiardjo / Jacqueline J Stevens / Anna L Calkins / Ayotunde P Ikujuni / Virangika K Wimalasena / Emre Firlar / David A Case / Julie S Biteen / Jason T Kaelber / Joanna S G Slusky /  要旨: The double membrane architecture of Gram-negative bacteria forms a barrier that is impermeable to most extracellular threats. Bacteriocin proteins evolved to exploit the accessible, surface-exposed ...The double membrane architecture of Gram-negative bacteria forms a barrier that is impermeable to most extracellular threats. Bacteriocin proteins evolved to exploit the accessible, surface-exposed proteins embedded in the outer membrane to deliver cytotoxic cargo. Colicin E1 is a bacteriocin produced by, and lethal to, that hijacks the outer membrane proteins (OMPs) TolC and BtuB to enter the cell. Here, we capture the colicin E1 translocation domain inside its membrane receptor, TolC, by high-resolution cryo-electron microscopy to obtain the first reported structure of a bacteriocin bound to TolC. Colicin E1 binds stably to TolC as an open hinge through the TolC pore-an architectural rearrangement from colicin E1's unbound conformation. This binding is stable in live cells as indicated by single-molecule fluorescence microscopy. Finally, colicin E1 fragments binding to TolC plug the channel, inhibiting its native efflux function as an antibiotic efflux pump, and heightening susceptibility to three antibiotic classes. In addition to demonstrating that these protein fragments are useful starting points for developing novel antibiotic potentiators, this method could be expanded to other colicins to inhibit other OMP functions. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_21959.map.gz emd_21959.map.gz | 52.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-21959-v30.xml emd-21959-v30.xml emd-21959.xml emd-21959.xml | 24.4 KB 24.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_21959_fsc.xml emd_21959_fsc.xml | 15.1 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_21959.png emd_21959.png | 64.1 KB | ||
| マスクデータ |  emd_21959_msk_1.map emd_21959_msk_1.map | 282.6 MB |  マスクマップ マスクマップ | |
| Filedesc metadata |  emd-21959.cif.gz emd-21959.cif.gz | 7.1 KB | ||
| その他 |  emd_21959_additional_1.map.gz emd_21959_additional_1.map.gz emd_21959_half_map_1.map.gz emd_21959_half_map_1.map.gz emd_21959_half_map_2.map.gz emd_21959_half_map_2.map.gz | 145.9 MB 262 MB 262 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21959 http://ftp.pdbj.org/pub/emdb/structures/EMD-21959 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21959 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21959 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_21959_validation.pdf.gz emd_21959_validation.pdf.gz | 1.1 MB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_21959_full_validation.pdf.gz emd_21959_full_validation.pdf.gz | 1.1 MB | 表示 | |
| XML形式データ |  emd_21959_validation.xml.gz emd_21959_validation.xml.gz | 22.5 KB | 表示 | |
| CIF形式データ |  emd_21959_validation.cif.gz emd_21959_validation.cif.gz | 28.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21959 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21959 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21959 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21959 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_21959.map.gz / 形式: CCP4 / 大きさ: 67 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_21959.map.gz / 形式: CCP4 / 大きさ: 67 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | asymmetric map of colicin E1 bound to TolC in nanodisc; cropped, sharpened, and resampled | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
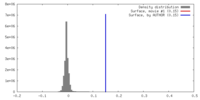
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-マスク #1
| ファイル |  emd_21959_msk_1.map emd_21959_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
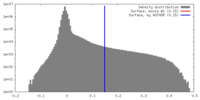
| 密度ヒストグラム |
-追加マップ: C3-symmetrized refinement of colicin E1 bound to TolC...
| ファイル | emd_21959_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | C3-symmetrized refinement of colicin E1 bound to TolC in nanodisc; sharpened | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
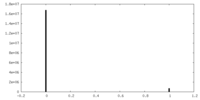
| 密度ヒストグラム |
-ハーフマップ: unmasked, unfiltered cryoSPARC half-map B of colicin E1...
| ファイル | emd_21959_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | unmasked, unfiltered cryoSPARC half-map B of colicin E1 bound to TolC in nanodisc | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
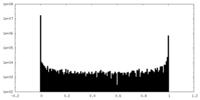
| 密度ヒストグラム |
-ハーフマップ: unmasked, unfiltered cryoSPARC half-map A of colicin E1...
| ファイル | emd_21959_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | unmasked, unfiltered cryoSPARC half-map A of colicin E1 bound to TolC in nanodisc | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Colicin E1 fragment colE1-T inside nanodisc-embedded TolC
| 全体 | 名称: Colicin E1 fragment colE1-T inside nanodisc-embedded TolC |
|---|---|
| 要素 |
|
-超分子 #1: Colicin E1 fragment colE1-T inside nanodisc-embedded TolC
| 超分子 | 名称: Colicin E1 fragment colE1-T inside nanodisc-embedded TolC タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 分子量 | 理論値: 21.44974 KDa |
-超分子 #2: Outer membrane protein TolC
| 超分子 | 名称: Outer membrane protein TolC / タイプ: complex / ID: 2 / 親要素: 1 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  |
-超分子 #3: Colicin E1 fragment colE1-T
| 超分子 | 名称: Colicin E1 fragment colE1-T / タイプ: complex / ID: 3 / 親要素: 1 / 含まれる分子: #2 |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Outer membrane protein TolC
| 分子 | 名称: Outer membrane protein TolC / タイプ: protein_or_peptide / ID: 1 / コピー数: 3 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 53.783355 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MKKLLPILIG LSLSGFSSLS QAENLMQVYQ QARLSNPELR KSAADRDAAF EKINEARSPL LPQLGLGADY TYSNGYRDAN GINSNATSA SLQLTQSIFD MSKWRALTLQ EKAAGIQDVT YQTDQQTLIL NTATAYFNVL NAIDVLSYTQ AQKEAIYRQL D QTTQRFNV ...文字列: MKKLLPILIG LSLSGFSSLS QAENLMQVYQ QARLSNPELR KSAADRDAAF EKINEARSPL LPQLGLGADY TYSNGYRDAN GINSNATSA SLQLTQSIFD MSKWRALTLQ EKAAGIQDVT YQTDQQTLIL NTATAYFNVL NAIDVLSYTQ AQKEAIYRQL D QTTQRFNV GLVAITDVQN ARAQYDTVLA NEVTARNNLD NAVEQLRQIT GNYYPELAAL NVENFKTDKP QPVNALLKEA EK RNLSLLQ ARLSQDLARE QIRQAQDGHL PTLDLTASTG ISDTSYSGSK TRGAAGTQYD DSNMGQNKVG LSFSLPIYQG GMV NSQVKQ AQYNFVGASE QLESAHRSVV QTVRSSFNNI NASISSINAY KQAVVSAQSS LDAMEAGYSV GTRTIVDVLD ATTT LYNAK QELANARYNY LINQLNIKSA LGTLNEQDLL ALNNALSKPV STNPENVAPQ TPEQNAIADG YAPDSPAPVV QQTSA RTTT SNGHNPFRN UniProtKB: Outer membrane protein TolC |
-分子 #2: Colicin-E1
| 分子 | 名称: Colicin-E1 / タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 21.491807 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: METAVAYYKD GVPYDDKGQV IITLLNGTPD GSGSGGGGGK GGSKSESSAA IHATAKWSTA QLKKTQAEQA ARAKAAAEAQ AKAKANRDA LTQRLKDIVN EALRHNASRT PSATELAHAN NAAMQAEDER LRLAKAEEKA RKEAEAAEKA FQEAEQRRKE I EREKAETE ...文字列: METAVAYYKD GVPYDDKGQV IITLLNGTPD GSGSGGGGGK GGSKSESSAA IHATAKWSTA QLKKTQAEQA ARAKAAAEAQ AKAKANRDA LTQRLKDIVN EALRHNASRT PSATELAHAN NAAMQAEDER LRLAKAEEKA RKEAEAAEKA FQEAEQRRKE I EREKAETE RQLKLAEAEE KRLAALSEEA KALEHHHHHH UniProtKB: Colicin-E1 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 8 構成要素:
| |||||||||
| グリッド | モデル: UltrAuFoil / 材質: GOLD / メッシュ: 200 / 支持フィルム - 材質: GOLD / 支持フィルム - トポロジー: HOLEY ARRAY / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 雰囲気: AIR | |||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 298 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TALOS ARCTICA |
|---|---|
| 特殊光学系 | エネルギーフィルター - 名称: GIF Quantum LS / エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: COUNTING / デジタル化 - サイズ - 横: 3838 pixel / デジタル化 - サイズ - 縦: 3710 pixel / 実像数: 10644 / 平均露光時間: 6.0 sec. / 平均電子線量: 35.96 e/Å2 / 詳細: 5 frames per second |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 20.0 µm / 最大 デフォーカス(補正後): 2.1 µm / 最小 デフォーカス(補正後): 0.4 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 倍率(公称値): 205000 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Talos Arctica / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)