[English] 日本語
 Yorodumi
Yorodumi- EMDB-21700: SD-like state of human 26S proteasome in complex with non-cleavab... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21700 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | SD-like state of human 26S proteasome in complex with non-cleavable M1-linked hexaubiquitin | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
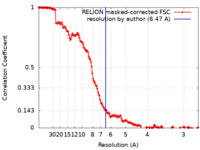
| Method | single particle reconstruction / cryo EM / Resolution: 6.47 Å | ||||||||||||
 Authors Authors | Chen X / Walters KJ | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Structure / Year: 2020 Journal: Structure / Year: 2020Title: Cryo-EM Reveals Unanchored M1-Ubiquitin Chain Binding at hRpn11 of the 26S Proteasome. Authors: Xiang Chen / Zachary Dorris / Dan Shi / Rick K Huang / Htet Khant / Tara Fox / Natalia de Val / Dewight Williams / Ping Zhang / Kylie J Walters /  Abstract: The 26S proteasome is specialized for regulated protein degradation and formed by a dynamic regulatory particle (RP) that caps a hollow cylindrical core particle (CP) where substrates are proteolyzed. ...The 26S proteasome is specialized for regulated protein degradation and formed by a dynamic regulatory particle (RP) that caps a hollow cylindrical core particle (CP) where substrates are proteolyzed. Its diverse substrates unify as proteasome targets by ubiquitination. We used cryogenic electron microscopy (cryo-EM) to study how human 26S proteasome interacts with M1-linked hexaubiquitin (M1-Ub) unanchored to a substrate and E3 ubiquitin ligase E6AP/UBE3A. Proteasome structures are available with model substrates extending through the RP ATPase ring and substrate-conjugated K63-linked ubiquitin chains present at inhibited deubiquitinating enzyme hRpn11 and the nearby ATPase hRpt4/hRpt5 coiled coil. In this study, we find M1-Ub at the hRpn11 site despite the absence of conjugated substrate, indicating that ubiquitin binding at this location does not require substrate interaction with the RP. Moreover, unanchored M1-Ub binds to this hRpn11 site of the proteasome with the CP gating residues in both the closed and opened conformational states. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21700.map.gz emd_21700.map.gz | 23.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21700-v30.xml emd-21700-v30.xml emd-21700.xml emd-21700.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21700_fsc.xml emd_21700_fsc.xml | 16 KB | Display |  FSC data file FSC data file |
| Images |  emd_21700.png emd_21700.png | 109.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21700 http://ftp.pdbj.org/pub/emdb/structures/EMD-21700 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21700 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21700 | HTTPS FTP |
-Validation report
| Summary document |  emd_21700_validation.pdf.gz emd_21700_validation.pdf.gz | 78.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21700_full_validation.pdf.gz emd_21700_full_validation.pdf.gz | 77.2 KB | Display | |
| Data in XML |  emd_21700_validation.xml.gz emd_21700_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21700 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21700 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21700 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21700 | HTTPS FTP |
-Related structure data
| Related structure data |  6wjdC  6wjnC C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10403 (Title: SA-like and SD-like states of human 26S Proteasome with non-cleavable M1-linked hexaubiquitin (dataset 1) EMPIAR-10403 (Title: SA-like and SD-like states of human 26S Proteasome with non-cleavable M1-linked hexaubiquitin (dataset 1)Data size: 87.3 Data #1: Motion-corrected single frame micrographs of human 26S Proteasome with non-cleavable M1-linked hexaubiquitin (dataset 1) [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21700.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21700.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.365 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human 26S Proteasome with non-cleavable M1-linked hexaubiquitin
| Entire | Name: Human 26S Proteasome with non-cleavable M1-linked hexaubiquitin |
|---|---|
| Components |
|
-Supramolecule #1: Human 26S Proteasome with non-cleavable M1-linked hexaubiquitin
| Supramolecule | Name: Human 26S Proteasome with non-cleavable M1-linked hexaubiquitin type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 50 mM Tris, pH 7.5, 50 mM NaCl, 1.5 mM ATP-gamma-S, 5 mM MgCl2, 2 mM DTT, 10 uM zinc sulfate | |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 291.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 1645 / Average exposure time: 0.2 sec. / Average electron dose: 43.44 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)