[English] 日本語
 Yorodumi
Yorodumi- EMDB-21673: GluN1b-GluN2B NMDA receptor in non-active 2 conformation at 4 ang... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21673 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GluN1b-GluN2B NMDA receptor in non-active 2 conformation at 4 angstrom resolution | |||||||||
 Map data Map data | B_factor sharpened map (-90) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NMDARs / Ligand-gated ion channels / METAL TRANSPORT / Ionotropic glutamate receptor / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / pons maturation / positive regulation of Schwann cell migration / regulation of cell communication / sensitization / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors ...cellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / pons maturation / positive regulation of Schwann cell migration / regulation of cell communication / sensitization / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors / response to hydrogen sulfide / auditory behavior / olfactory learning / conditioned taste aversion / dendritic branch / regulation of respiratory gaseous exchange / response to other organism / protein localization to postsynaptic membrane / regulation of ARF protein signal transduction / fear response / apical dendrite / transmitter-gated monoatomic ion channel activity / positive regulation of inhibitory postsynaptic potential / suckling behavior / response to methylmercury / response to glycine / propylene metabolic process / response to manganese ion / response to carbohydrate / interleukin-1 receptor binding / cellular response to dsRNA / response to growth hormone / cellular response to lipid / negative regulation of dendritic spine maintenance / heterocyclic compound binding / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / positive regulation of glutamate secretion / RAF/MAP kinase cascade / Synaptic adhesion-like molecules / voltage-gated monoatomic cation channel activity / response to glycoside / NMDA selective glutamate receptor complex / glutamate binding / ligand-gated sodium channel activity / neurotransmitter receptor complex / response to morphine / calcium ion transmembrane import into cytosol / regulation of axonogenesis / neuromuscular process / regulation of dendrite morphogenesis / protein heterotetramerization / male mating behavior / glycine binding / regulation of synapse assembly / response to amine / small molecule binding / regulation of cAMP/PKA signal transduction / parallel fiber to Purkinje cell synapse / receptor clustering / startle response / positive regulation of reactive oxygen species biosynthetic process / monoatomic cation transmembrane transport / behavioral response to pain / positive regulation of calcium ion transport into cytosol / regulation of MAPK cascade / response to magnesium ion / regulation of postsynaptic membrane potential / cellular response to glycine / associative learning / action potential / extracellularly glutamate-gated ion channel activity / excitatory synapse / monoatomic cation transport / positive regulation of dendritic spine maintenance / response to electrical stimulus / social behavior / regulation of neuronal synaptic plasticity / monoatomic ion channel complex / glutamate receptor binding / positive regulation of excitatory postsynaptic potential / Unblocking of NMDA receptors, glutamate binding and activation / long-term memory / response to mechanical stimulus / detection of mechanical stimulus involved in sensory perception of pain / synaptic cleft / neuron development / positive regulation of synaptic transmission, glutamatergic / behavioral fear response / prepulse inhibition / phosphatase binding / multicellular organismal response to stress / postsynaptic density, intracellular component / monoatomic cation channel activity / calcium ion homeostasis / response to fungicide / glutamate-gated receptor activity / cell adhesion molecule binding / regulation of neuron apoptotic process / D2 dopamine receptor binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.99 Å | |||||||||
 Authors Authors | Chou T / Tajima N | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: Structural Basis of Functional Transitions in Mammalian NMDA Receptors. Authors: Tsung-Han Chou / Nami Tajima / Annabel Romero-Hernandez / Hiro Furukawa /  Abstract: Excitatory neurotransmission meditated by glutamate receptors including N-methyl-D-aspartate receptors (NMDARs) is pivotal to brain development and function. NMDARs are heterotetramers composed of ...Excitatory neurotransmission meditated by glutamate receptors including N-methyl-D-aspartate receptors (NMDARs) is pivotal to brain development and function. NMDARs are heterotetramers composed of GluN1 and GluN2 subunits, which bind glycine and glutamate, respectively, to activate their ion channels. Despite importance in brain physiology, the precise mechanisms by which activation and inhibition occur via subunit-specific binding of agonists and antagonists remain largely unknown. Here, we show the detailed patterns of conformational changes and inter-subunit and -domain reorientation leading to agonist-gating and subunit-dependent competitive inhibition by providing multiple structures in distinct ligand states at 4 Å or better. The structures reveal that activation and competitive inhibition by both GluN1 and GluN2 antagonists occur by controlling the tension of the linker between the ligand-binding domain and the transmembrane ion channel of the GluN2 subunit. Our results provide detailed mechanistic insights into NMDAR pharmacology, activation, and inhibition, which are fundamental to the brain physiology. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21673.map.gz emd_21673.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21673-v30.xml emd-21673-v30.xml emd-21673.xml emd-21673.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21673.png emd_21673.png | 61.7 KB | ||
| Filedesc metadata |  emd-21673.cif.gz emd-21673.cif.gz | 6.7 KB | ||
| Others |  emd_21673_additional.map.gz emd_21673_additional.map.gz | 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21673 http://ftp.pdbj.org/pub/emdb/structures/EMD-21673 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21673 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21673 | HTTPS FTP |
-Validation report
| Summary document |  emd_21673_validation.pdf.gz emd_21673_validation.pdf.gz | 576.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21673_full_validation.pdf.gz emd_21673_full_validation.pdf.gz | 576 KB | Display | |
| Data in XML |  emd_21673_validation.xml.gz emd_21673_validation.xml.gz | 6.4 KB | Display | |
| Data in CIF |  emd_21673_validation.cif.gz emd_21673_validation.cif.gz | 7.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21673 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21673 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21673 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21673 | HTTPS FTP |
-Related structure data
| Related structure data |  6whrMC  6usuC  6usvC  6whsC  6whtC  6whuC  6whvC  6whwC  6whxC  6whyC  6wi0C  6wi1C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21673.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21673.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B_factor sharpened map (-90) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
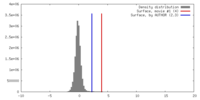
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unsharpened map
| File | emd_21673_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
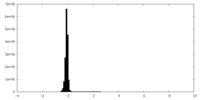
| Density Histograms |
- Sample components
Sample components
-Entire : NMDA receptor GluN1b/2B functional ion channel complex
| Entire | Name: NMDA receptor GluN1b/2B functional ion channel complex |
|---|---|
| Components |
|
-Supramolecule #1: NMDA receptor GluN1b/2B functional ion channel complex
| Supramolecule | Name: NMDA receptor GluN1b/2B functional ion channel complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamate receptor ionotropic, NMDA 1
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 108.085633 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSTMHLLTFA LLFSCSFARA ASDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL QATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYN W NHIILLVS ...String: MSTMHLLTFA LLFSCSFARA ASDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL QATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYN W NHIILLVS DDHEGRAAQK RLETLLEERE SKSKKRNYEN LDQLSYDNKR GPKAEKVLQF DPGTKNVTAL LMEARELEAR VI ILSASED DAATVYRAAA MLDMTGSGYV WLVGEREISG NALRYAPDGI IGLQLINGKN ESAHISDAVG VVAQAVHELL EKE NITDPP RGCVGNTNIW KTGPLFKRVL MSSKYADGVT GRVEFNEDGD RKFAQYSIMN LQNRKLVQVG IYNGTHVIPN DRKI IWPGG ETEKPRGYQM STRLKIVTIH QEPFVYVKPT MSDGTCKEEF TVNGDPVKKV ICTGPNDTSP GSPRHTVPQC CYGFC IDLL IKLARTMQFT YEVHLVADGK FGTQERVQNS NKKEWNGMMG ELLSGQADMI VAPLTINNER AQYIEFSKPF KYQGLT ILV KKEIPRSTLD SFMQPFQSTL WLLVGLSVHV VAVMLYLLDR FSPFGRFKVN SQSESTDALT LSSAMWFSWG VLLNSGI GE GAPRSFSARI LGMVWAGFAM IIVASYTANL AAFLVLDRPE ERITGINDPR LRNPSDKFIY ATVKQSSVDI YFRRQVEL S TMYRHMEKHN YESAAEAIQA VRDNKLHAFI WDSAVLEFEA SQKCDLVTTG ELFFRSGFGI GMRKDSPWKQ QVSLSILKS HENGFMEDLD KTWVRYQECD SRSNAPATLT CENMAGVFML VAGGIVAGIF LIFIEIAYKR HKDARRKQMQ LAFAAVNVWR KNLQDRKSG RAEPDPKKKA TFRAITSTLA SSFKRRRSSK DTSTGGGRGA LQNQKDTVLP RRAIEREEGQ LQLCSRHRES UniProtKB: Glutamate receptor ionotropic, NMDA 1 |
-Macromolecule #2: Glutamate receptor ionotropic, NMDA 2B
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 2B / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.845859 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGTMRLFLLA VLFLFSFARA TGWSHPQFEK GGGSGGGSGG SAWSHPQFEK GALVPRGRSQ KSPPSIGIAV ILVGTSDEVA IKDAHEKDD FHHLSVVPRV ELVAMNETDP KSIITRICDL MSDRKIQGVV FADDTDQEAI AQILDFISAQ TLTPILGIHG G SSMIMADK ...String: MGTMRLFLLA VLFLFSFARA TGWSHPQFEK GGGSGGGSGG SAWSHPQFEK GALVPRGRSQ KSPPSIGIAV ILVGTSDEVA IKDAHEKDD FHHLSVVPRV ELVAMNETDP KSIITRICDL MSDRKIQGVV FADDTDQEAI AQILDFISAQ TLTPILGIHG G SSMIMADK DESSMFFQFG PSIEQQASVM LNIMEEYDWY IFSIVTTYFP GYQDFVNKIR STIENSFVGW ELEEVLLLDM SL DDGDSKI QNQLKKLQSP IILLYCTKEE ATYIFEVANS VGLTGYGYTW IVPSLVAGDT DTVPSEFPTG LISVSYDEWD YGL PARVRD GIAIITTAAS DMLSEHSFIP EPKSSCYNTH EKRIYQSNML NRYLINVTFE GRDLSFSEDG YQMHPKLVII LLNK ERKWE RVGKWKDKSL QMKYYVWPRM CPETEEQEDD HLSIVTLEEA PFVIVESVDP LSGTCMRNTV PCQKRIISEN KTDEE PGYI KKCCKGFCID ILKKISKSVK FTYDLYLVTN GKHGKKINGT WNGMIGEVVM KRAYMAVGSL TINEERSEVV DFSVPF IET GISVMVSRSN GTVSPSAFLE PFSACVWVMM FVMLLIVSAV AVFVFEYFSP VGYNRSLADG REPGGPSFTI GKAIWLL WG LVFNNSVPVQ NPKGTTSKIM VSVWAFFAVI FLASYTANLA AFMIQEEYVD QVSGLSDKKF QRPNDFSPPF RFGTVPNG S TERNIRNNYA EMHAYMGKFN QRGVDDALLS LKTGKLDAFI YDAAVLNYMA GRDEGCKLVT IGSGKVFAST GYGIAIQKD SGWKRQVDLA ILQLFGDGEM EELEALWLTG ICHNEKNEVM SSQLDIDNMA GVFYMLGAAM ALSLITFISE HLFYWQFRHS FMG UniProtKB: Glutamate receptor ionotropic, NMDA 2B |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 10 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Pretreatment - Type: GLOW DISCHARGE / Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 64.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: Ab initio reconstruction |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 3.99 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cisTEM (ver. 1.0.0) / Number images used: 134688 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)