+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21652 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | NanR dimer-DNA hetero-complex | |||||||||
 Map data Map data | Relion local resolution filtered map. Sharpening B-factor -100 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NanR dimer-DNA hetero-complex / transcriptional regulator / GntR superfamily / sialic acid / Neu5Ac / cooperativity. / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA-binding transcription factor activity / negative regulation of DNA-templated transcription / DNA binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
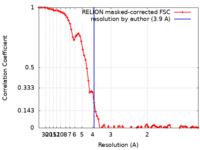
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Hariprasad V / Horne C | |||||||||
| Funding support |  New Zealand, 1 items New Zealand, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Mechanism of NanR gene repression and allosteric induction of bacterial sialic acid metabolism. Authors: Christopher R Horne / Hariprasad Venugopal / Santosh Panjikar / David M Wood / Amy Henrickson / Emre Brookes / Rachel A North / James M Murphy / Rosmarie Friemann / Michael D W Griffin / ...Authors: Christopher R Horne / Hariprasad Venugopal / Santosh Panjikar / David M Wood / Amy Henrickson / Emre Brookes / Rachel A North / James M Murphy / Rosmarie Friemann / Michael D W Griffin / Georg Ramm / Borries Demeler / Renwick C J Dobson /      Abstract: Bacteria respond to environmental changes by inducing transcription of some genes and repressing others. Sialic acids, which coat human cell surfaces, are a nutrient source for pathogenic and ...Bacteria respond to environmental changes by inducing transcription of some genes and repressing others. Sialic acids, which coat human cell surfaces, are a nutrient source for pathogenic and commensal bacteria. The Escherichia coli GntR-type transcriptional repressor, NanR, regulates sialic acid metabolism, but the mechanism is unclear. Here, we demonstrate that three NanR dimers bind a (GGTATA)-repeat operator cooperatively and with high affinity. Single-particle cryo-electron microscopy structures reveal the DNA-binding domain is reorganized to engage DNA, while three dimers assemble in close proximity across the (GGTATA)-repeat operator. Such an interaction allows cooperative protein-protein interactions between NanR dimers via their N-terminal extensions. The effector, N-acetylneuraminate, binds NanR and attenuates the NanR-DNA interaction. The crystal structure of NanR in complex with N-acetylneuraminate reveals a domain rearrangement upon N-acetylneuraminate binding to lock NanR in a conformation that weakens DNA binding. Our data provide a molecular basis for the regulation of bacterial sialic acid metabolism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21652.map.gz emd_21652.map.gz | 31.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21652-v30.xml emd-21652-v30.xml emd-21652.xml emd-21652.xml | 23.8 KB 23.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21652_fsc.xml emd_21652_fsc.xml | 8.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_21652.png emd_21652.png | 158.5 KB | ||
| Masks |  emd_21652_msk_1.map emd_21652_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21652.cif.gz emd-21652.cif.gz | 6.6 KB | ||
| Others |  emd_21652_additional_1.map.gz emd_21652_additional_1.map.gz emd_21652_additional_2.map.gz emd_21652_additional_2.map.gz emd_21652_half_map_1.map.gz emd_21652_half_map_1.map.gz emd_21652_half_map_2.map.gz emd_21652_half_map_2.map.gz | 32.4 MB 7.1 MB 33 MB 33 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21652 http://ftp.pdbj.org/pub/emdb/structures/EMD-21652 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21652 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21652 | HTTPS FTP |
-Related structure data
| Related structure data |  6wfqMC  6wg7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10836 (Title: CryoEM single particle dataset for NanR dimer-DNA hetero-complex. EMPIAR-10836 (Title: CryoEM single particle dataset for NanR dimer-DNA hetero-complex.Data size: 2.9 TB Data #1: Uncorrected movie frames [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21652.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21652.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Relion local resolution filtered map. Sharpening B-factor -100 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.68 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21652_msk_1.map emd_21652_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #2
| File | emd_21652_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_21652_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_21652_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_21652_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : NanR-DNA hetero-complex
| Entire | Name: NanR-DNA hetero-complex |
|---|---|
| Components |
|
-Supramolecule #1: NanR-DNA hetero-complex
| Supramolecule | Name: NanR-DNA hetero-complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Dimeric NanR-DNA hetero-complex |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 72.8 KDa |
-Macromolecule #1: HTH-type transcriptional repressor NanR
| Macromolecule | Name: HTH-type transcriptional repressor NanR / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.566354 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGLMNAFDSQ TEDSSPAIGR NLRSRPLARK KLSEMVEEEL EQMIRRREFG EGEQLPSERE LMAFFNVGRP SVREALAALK RKGLVQINN GERARVSRPS ADTIIGELSG MAKDFLSHPG GIAHFEQLRL FFESSLVRYA AEHATDEQID LLAKALEINS Q SLDNNAAF ...String: MGLMNAFDSQ TEDSSPAIGR NLRSRPLARK KLSEMVEEEL EQMIRRREFG EGEQLPSERE LMAFFNVGRP SVREALAALK RKGLVQINN GERARVSRPS ADTIIGELSG MAKDFLSHPG GIAHFEQLRL FFESSLVRYA AEHATDEQID LLAKALEINS Q SLDNNAAF IRSDVDFHRV LAEIPGNPIF MAIHVALLDW LIAARPTVTD QALHEHNNVS YQQHIAIVDA IRRHDPDEAD RA LQSHLNS VSATWHAFGQ TTNKKK UniProtKB: HTH-type transcriptional repressor NanR |
-Macromolecule #2: DNA (5'-D(P*GP*GP*TP*AP*TP*AP*AP*CP*AP*GP*GP*TP*AP*TP*A)-3')
| Macromolecule | Name: DNA (5'-D(P*GP*GP*TP*AP*TP*AP*AP*CP*AP*GP*GP*TP*AP*TP*A)-3') type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.65706 KDa |
| Sequence | String: (DG)(DG)(DT)(DA)(DT)(DA)(DA)(DC)(DA)(DG) (DG)(DT)(DA)(DT)(DA) |
-Macromolecule #3: DNA (5'-D(P*TP*AP*TP*AP*CP*CP*TP*GP*TP*TP*AP*TP*AP*CP*C)-3')
| Macromolecule | Name: DNA (5'-D(P*TP*AP*TP*AP*CP*CP*TP*GP*TP*TP*AP*TP*AP*CP*C)-3') type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.518959 KDa |
| Sequence | String: (DT)(DA)(DT)(DA)(DC)(DC)(DT)(DG)(DT)(DT) (DA)(DT)(DA)(DC)(DC) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 20mM Tris-HCL, 150mM NaCl, 100 microM ZnCl2, pH 8.0 | |||||||||
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK III Details: blotting conditions: 3 sec blotting time and -3 blot force.. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-20 / Number grids imaged: 1 / Number real images: 3465 / Average exposure time: 12.8 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 215000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6wfq: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)