[English] 日本語
 Yorodumi
Yorodumi- EMDB-21147: Cryo-EM structure of the Glucagon-like peptide-1 receptor in comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21147 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Glucagon-like peptide-1 receptor in complex with G protein, GLP-1 peptide and a positive allosteric modulator | |||||||||
 Map data Map data | Glucagon-like peptide-1 receptor in complex with G protein, GLP-1 peptide and a positive allosteric modulator | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | G protein-coupled receptor / membrane protein / family B GPCR / diabetes / allosteric modulator / SIGNALING PROTEIN-MEMBRANE PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationglucagon receptor binding / glucagon-like peptide 1 receptor activity / glucagon receptor activity / : / positive regulation of blood pressure / hormone secretion / post-translational protein targeting to membrane, translocation / negative regulation of execution phase of apoptosis / feeding behavior / response to psychosocial stress ...glucagon receptor binding / glucagon-like peptide 1 receptor activity / glucagon receptor activity / : / positive regulation of blood pressure / hormone secretion / post-translational protein targeting to membrane, translocation / negative regulation of execution phase of apoptosis / feeding behavior / response to psychosocial stress / positive regulation of calcium ion import / regulation of heart contraction / peptide hormone binding / PKA activation in glucagon signalling / Synthesis, secretion, and deacylation of Ghrelin / developmental growth / hair follicle placode formation / intracellular transport / D1 dopamine receptor binding / positive regulation of insulin secretion involved in cellular response to glucose stimulus / vascular endothelial cell response to laminar fluid shear stress / activation of adenylate cyclase activity / renal water homeostasis / Hedgehog 'off' state / negative regulation of blood pressure / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of insulin secretion / cellular response to glucagon stimulus / positive regulation of gluconeogenesis / adenylate cyclase activator activity / trans-Golgi network membrane / response to activity / gluconeogenesis / negative regulation of inflammatory response to antigenic stimulus / hormone activity / bone development / platelet aggregation / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cognition / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / sensory perception of smell / Vasopressin regulates renal water homeostasis via Aquaporins / transmembrane signaling receptor activity / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / glucose homeostasis / extracellular vesicle / sensory perception of taste / positive regulation of cold-induced thermogenesis / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / positive regulation of cytosolic calcium ion concentration / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / secretory granule lumen / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction / learning or memory / cell surface receptor signaling pathway / Extra-nuclear estrogen signaling Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Sun B / Feng D | |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2020 Journal: Nat Chem Biol / Year: 2020Title: Structural insights into probe-dependent positive allosterism of the GLP-1 receptor. Authors: Ana B Bueno / Bingfa Sun / Francis S Willard / Dan Feng / Joseph D Ho / David B Wainscott / Aaron D Showalter / Michal Vieth / Qi Chen / Cynthia Stutsman / Betty Chau / James Ficorilli / ...Authors: Ana B Bueno / Bingfa Sun / Francis S Willard / Dan Feng / Joseph D Ho / David B Wainscott / Aaron D Showalter / Michal Vieth / Qi Chen / Cynthia Stutsman / Betty Chau / James Ficorilli / Francisco J Agejas / Graham R Cumming / Alma Jiménez / Isabel Rojo / Tong Sun Kobilka / Brian K Kobilka / Kyle W Sloop /   Abstract: Drugs that promote the association of protein complexes are an emerging therapeutic strategy. We report discovery of a G protein-coupled receptor (GPCR) ligand that stabilizes an active state ...Drugs that promote the association of protein complexes are an emerging therapeutic strategy. We report discovery of a G protein-coupled receptor (GPCR) ligand that stabilizes an active state conformation by cooperatively binding both the receptor and orthosteric ligand, thereby acting as a 'molecular glue'. LSN3160440 is a positive allosteric modulator of the GLP-1R optimized to increase the affinity and efficacy of GLP-1(9-36), a proteolytic product of GLP-1(7-36). The compound enhances insulin secretion in a glucose-, ligand- and GLP-1R-dependent manner. Cryo-electron microscopy determined the structure of the GLP-1R bound to LSN3160440 in complex with GLP-1 and heterotrimeric G. The modulator binds high in the helical bundle at an interface between TM1 and TM2, allowing access to the peptide ligand. Pharmacological characterization showed strong probe dependence of LSN3160440 for GLP-1(9-36) versus oxyntomodulin that is driven by a single residue. Our findings expand protein-protein modulation drug discovery to uncompetitive, active state stabilizers for peptide hormone receptors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21147.map.gz emd_21147.map.gz | 49.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21147-v30.xml emd-21147-v30.xml emd-21147.xml emd-21147.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21147.png emd_21147.png | 43.7 KB | ||
| Filedesc metadata |  emd-21147.cif.gz emd-21147.cif.gz | 7.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21147 http://ftp.pdbj.org/pub/emdb/structures/EMD-21147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21147 | HTTPS FTP |
-Related structure data
| Related structure data |  6vcbMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21147.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21147.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Glucagon-like peptide-1 receptor in complex with G protein, GLP-1 peptide and a positive allosteric modulator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
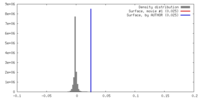
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Cryo-EM structure of the Glucagon-like peptide-1 receptor in comp...
+Supramolecule #1: Cryo-EM structure of the Glucagon-like peptide-1 receptor in comp...
+Supramolecule #3: Guanine nucleotide-binding protein G(s) subunit alpha isoforms sh...
+Supramolecule #2: Glucagon-like peptide 1 receptor, Glucagon-like peptide 1
+Supramolecule #4: Nanobody 35
+Macromolecule #1: Glucagon-like peptide 1 receptor
+Macromolecule #2: Glucagon-like peptide 1
+Macromolecule #3: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
+Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #6: Nanobody 35
+Macromolecule #7: 1-[(1R)-1-(2,6-dichloro-3-methoxyphenyl)ethyl]-6-{2-[(2R)-piperid...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 200 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 10.8 sec. / Average electron dose: 81.56 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 323427 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Protocol: FLEXIBLE FIT / Overall B value: 72.5 |
| Output model |  PDB-6vcb: |
 Movie
Movie Controller
Controller



































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)