+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20434 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ClpP and ClpX IGF loop in ClpX-ClpP complex with D7 symmetry | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Protein degradation / AAA+ protease complex / CHAPERONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationHslUV protease complex / endopeptidase Clp / endopeptidase Clp complex / positive regulation of programmed cell death / response to temperature stimulus / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / protein unfolding / proteasomal protein catabolic process / : ...HslUV protease complex / endopeptidase Clp / endopeptidase Clp complex / positive regulation of programmed cell death / response to temperature stimulus / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / protein unfolding / proteasomal protein catabolic process / : / serine-type peptidase activity / ATP-dependent protein folding chaperone / response to radiation / disordered domain specific binding / unfolded protein binding / peptidase activity / protein folding / response to heat / ATPase binding / protease binding / protein dimerization activity / serine-type endopeptidase activity / cell division / ATP hydrolysis activity / zinc ion binding / ATP binding / identical protein binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
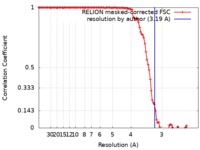
| Method | single particle reconstruction / cryo EM / Resolution: 3.19 Å | |||||||||
 Authors Authors | Fei X / Jenni S | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Structures of the ATP-fueled ClpXP proteolytic machine bound to protein substrate. Authors: Xue Fei / Tristan A Bell / Simon Jenni / Benjamin M Stinson / Tania A Baker / Stephen C Harrison / Robert T Sauer /  Abstract: ClpXP is an ATP-dependent protease in which the ClpX AAA+ motor binds, unfolds, and translocates specific protein substrates into the degradation chamber of ClpP. We present cryo-EM studies of the ...ClpXP is an ATP-dependent protease in which the ClpX AAA+ motor binds, unfolds, and translocates specific protein substrates into the degradation chamber of ClpP. We present cryo-EM studies of the enzyme that show how asymmetric hexameric rings of ClpX bind symmetric heptameric rings of ClpP and interact with protein substrates. Subunits in the ClpX hexamer assume a spiral conformation and interact with two-residue segments of substrate in the axial channel, as observed for other AAA+ proteases and protein-remodeling machines. Strictly sequential models of ATP hydrolysis and a power stroke that moves two residues of the substrate per translocation step have been inferred from these structural features for other AAA+ unfoldases, but biochemical and single-molecule biophysical studies indicate that ClpXP operates by a probabilistic mechanism in which five to eight residues are translocated for each ATP hydrolyzed. We propose structure-based models that could account for the functional results. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20434.map.gz emd_20434.map.gz | 48.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20434-v30.xml emd-20434-v30.xml emd-20434.xml emd-20434.xml | 16.5 KB 16.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20434_fsc.xml emd_20434_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_20434.png emd_20434.png | 72.5 KB | ||
| Filedesc metadata |  emd-20434.cif.gz emd-20434.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20434 http://ftp.pdbj.org/pub/emdb/structures/EMD-20434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20434 | HTTPS FTP |
-Related structure data
| Related structure data |  6ppeMC  6po1C  6po3C  6podC  6posC  6pp5C  6pp6C  6pp7C  6pp8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20434.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20434.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : ClpX-ClpP-substrate-ATPrS
| Entire | Name: ClpX-ClpP-substrate-ATPrS |
|---|---|
| Components |
|
-Supramolecule #1: ClpX-ClpP-substrate-ATPrS
| Supramolecule | Name: ClpX-ClpP-substrate-ATPrS / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: ATP-dependent Clp protease proteolytic subunit
| Macromolecule | Name: ATP-dependent Clp protease proteolytic subunit / type: protein_or_peptide / ID: 1 / Number of copies: 14 / Enantiomer: LEVO / EC number: endopeptidase Clp |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.515785 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LVPMVIEQTS RGERSFDIYS RLLKERVIFL TGQVEDHMAN LIVAQMLFLE AENPEKDIYL YINSPGGVIT AGMSIYDTMQ FIKPDVSTI CMGQAASMGA FLLTAGAKGK RFCLPNSRVM IHQPLGGYQG QATDIEIHAR EILKVKGRMN ELMALHTGQS L EQIERDTE ...String: LVPMVIEQTS RGERSFDIYS RLLKERVIFL TGQVEDHMAN LIVAQMLFLE AENPEKDIYL YINSPGGVIT AGMSIYDTMQ FIKPDVSTI CMGQAASMGA FLLTAGAKGK RFCLPNSRVM IHQPLGGYQG QATDIEIHAR EILKVKGRMN ELMALHTGQS L EQIERDTE RDRFLSAPEA VEYGLVDSIL THRN UniProtKB: ATP-dependent Clp protease proteolytic subunit |
-Macromolecule #2: ATP-dependent Clp protease ATP-binding subunit ClpX
| Macromolecule | Name: ATP-dependent Clp protease ATP-binding subunit ClpX / type: protein_or_peptide / ID: 2 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.835129 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SALPTPHEIR NHLDDYVIGQ EQAKKVLAVA VYNHYKRLRN GDTSNGVELG KSNILLIGPT GSGKTLLAET LARLLDVPFT MADATTLTE AGYVGEDVEN IIQKLLQKSD YDVQKAQRGI VYIDQIDKIS RKSDNPSITR DVSGEGVQQA LLKLIEGTVA A VPPQGGRK ...String: SALPTPHEIR NHLDDYVIGQ EQAKKVLAVA VYNHYKRLRN GDTSNGVELG KSNILLIGPT GSGKTLLAET LARLLDVPFT MADATTLTE AGYVGEDVEN IIQKLLQKSD YDVQKAQRGI VYIDQIDKIS RKSDNPSITR DVSGEGVQQA LLKLIEGTVA A VPPQGGRK HPQQEFLQVD TSKILFICGG AFAGLDKVIS HRVETGSGIG FGATVKAKSD KASEGELLAQ VEPEDLIKFG LI PEFIGRL PVVATLNELS EEALIQILKE PKNALTKQYQ ALFNLEGVDL EFRDEALDAI AKKAMARKTG ARGLRSIVEA ALL DTMYDL PSMEDVEKVV IDESVIDGQS EPLLIYGKPE AQQASGEGGG TSG UniProtKB: ATP-dependent Clp protease ATP-binding subunit ClpX |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 14 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 298 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 56.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: -2.5 µm / Nominal defocus min: -0.8 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)