[English] 日本語
 Yorodumi
Yorodumi- EMDB-2016: CryoEM map of the p97-Ufd1-Npl4 complex in the single-binding-sit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM map of the p97-Ufd1-Npl4 complex in the single-binding-site conformation | |||||||||
 Map data Map data | p97-Ufd1-Npl4 complex in the single-binding-site conformation | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | p97 / Ufd1-Npl4 / asymmetric complex / ATPase | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 25.0 Å | |||||||||
 Authors Authors | Bebeacua C / Forster A / McKeown C / Meyer HH / Zhang X / Freemont PS | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2012 Journal: Proc Natl Acad Sci U S A / Year: 2012Title: Distinct conformations of the protein complex p97-Ufd1-Npl4 revealed by electron cryomicroscopy. Authors: Cecilia Bebeacua / Andreas Förster / Ciarán McKeown / Hemmo H Meyer / Xiaodong Zhang / Paul S Freemont /  Abstract: p97 is a key regulator of numerous cellular pathways and associates with ubiquitin-binding adaptors to remodel ubiquitin-modified substrate proteins. How adaptor binding to p97 is coordinated and how ...p97 is a key regulator of numerous cellular pathways and associates with ubiquitin-binding adaptors to remodel ubiquitin-modified substrate proteins. How adaptor binding to p97 is coordinated and how adaptors contribute to substrate remodeling is unclear. Here we present the 3D electron cryomicroscopy reconstructions of the major Ufd1-Npl4 adaptor in complex with p97. Our reconstructions show that p97-Ufd1-Npl4 is highly dynamic and that Ufd1-Npl4 assumes distinct positions relative to the p97 ring upon addition of nucleotide. Our results suggest a model for substrate remodeling by p97 and also explains how p97-Ufd1-Npl4 could form other complexes in a hierarchical model of p97-cofactor assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2016.map.gz emd_2016.map.gz | 668.1 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2016-v30.xml emd-2016-v30.xml emd-2016.xml emd-2016.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| Images |  emd2016fig.png emd2016fig.png | 103 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2016 http://ftp.pdbj.org/pub/emdb/structures/EMD-2016 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2016 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2016 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2016.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2016.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | p97-Ufd1-Npl4 complex in the single-binding-site conformation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.53 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
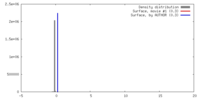
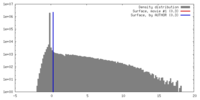
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : p97-Ufd1-Npl4
| Entire | Name: p97-Ufd1-Npl4 |
|---|---|
| Components |
|
-Supramolecule #1000: p97-Ufd1-Npl4
| Supramolecule | Name: p97-Ufd1-Npl4 / type: sample / ID: 1000 Oligomeric state: One hexamer of p97 binds to one Ufd1-Npl4 dimer Number unique components: 3 |
|---|---|
| Molecular weight | Experimental: 623 KDa / Theoretical: 623 KDa |
-Macromolecule #1: p97
| Macromolecule | Name: p97 / type: protein_or_peptide / ID: 1 / Name.synonym: p97 / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Macromolecule #2: Ufd1
| Macromolecule | Name: Ufd1 / type: protein_or_peptide / ID: 2 / Name.synonym: Ufd1 / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Macromolecule #3: Npl4
| Macromolecule | Name: Npl4 / type: protein_or_peptide / ID: 3 / Name.synonym: Npl4 / Number of copies: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 150 mM KCL, 25 mL Tris, 2.5 mM MgCl2 |
| Grid | Details: 200 mesh copper grid |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: OTHER / Details: Vitrification instrument: Vitrobot / Method: Blot for 2 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 100,000 times magnification |
| Specialist optics | Energy filter - Name: FEI |
| Date | Jul 1, 2009 |
| Image recording | Category: CCD / Film or detector model: GENERIC CCD / Number real images: 100 / Average electron dose: 10 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 25.0 Å / Resolution method: OTHER / Software - Name: IMAGIC / Number images used: 5000 |
|---|
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Protocol: Rigid Body. The protomers were fitted individually and the N-domains fitted by manual docking and refined automatically |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross Correlation |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)