+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the gTuRC-CM1dim complex | |||||||||
 Map data Map data | 3D Refinement map of the gTuRC-CM1dim complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | gTuRC / gTuSC / CM1 / CDK5RAP2 / gamma-tubulin / microtubule / alfa/beta-tubulin nucleation / cytoskeleton / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmicrotubule nucleation by interphase microtubule organizing center / gamma-tubulin complex localization / microtubule nucleator activity / polar microtubule / interphase microtubule organizing center / gamma-tubulin complex / gamma-tubulin ring complex / mitotic spindle microtubule / meiotic spindle organization / microtubule nucleation ...microtubule nucleation by interphase microtubule organizing center / gamma-tubulin complex localization / microtubule nucleator activity / polar microtubule / interphase microtubule organizing center / gamma-tubulin complex / gamma-tubulin ring complex / mitotic spindle microtubule / meiotic spindle organization / microtubule nucleation / gamma-tubulin binding / non-motile cilium / pericentriolar material / cell leading edge / microtubule organizing center / mitotic sister chromatid segregation / mitotic spindle assembly / single fertilization / cytoplasmic microtubule / spindle assembly / cytoplasmic microtubule organization / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / centriole / Recruitment of mitotic centrosome proteins and complexes / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / AURKA Activation by TPX2 / condensed nuclear chromosome / mitotic spindle organization / meiotic cell cycle / recycling endosome / brain development / structural constituent of cytoskeleton / microtubule cytoskeleton organization / spindle / neuron migration / apical part of cell / spindle pole / Regulation of PLK1 Activity at G2/M Transition / mitotic cell cycle / microtubule cytoskeleton / protein-containing complex assembly / microtubule binding / microtubule / neuron projection / cilium / ciliary basal body / centrosome / GTP binding / structural molecule activity / nucleoplasm / identical protein binding / nucleus / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||
 Authors Authors | Llorca O / Serna M / Gonzalez-Rodriguez N | |||||||||
| Funding support |  Spain, European Union, 2 items Spain, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: Dev Cell / Year: 2024 Journal: Dev Cell / Year: 2024Title: CDK5RAP2 activates microtubule nucleator γTuRC by facilitating template formation and actin release. Authors: Marina Serna / Fabian Zimmermann / Chithran Vineethakumari / Nayim Gonzalez-Rodriguez / Oscar Llorca / Jens Lüders /  Abstract: To organize microtubules, cells tightly control the activity of the microtubule nucleator γ-tubulin ring complex (γTuRC). The open ring-shaped γTuRC was proposed to nucleate microtubules by a ...To organize microtubules, cells tightly control the activity of the microtubule nucleator γ-tubulin ring complex (γTuRC). The open ring-shaped γTuRC was proposed to nucleate microtubules by a template mechanism. However, its splayed structure does not match microtubule symmetry, leaving it unclear how γTuRC becomes an efficient nucleator. Here, we identify the mechanism of γTuRC activation by CDK5RAP2 centrosomin motif 1 (CM1). Using cryoelectron microscopy (cryo-EM), we find that activation involves binding of multiple CM1 dimers to five distinct sites around the outside of the γTuRC cone, which crucially depends on regulatory modules formed by MZT2 and the N-terminal extensions of GCP2 subunits. CM1 binding promotes lateral interactions between GCP subunits to facilitate microtubule-like conformations and release of luminal actin that is integral to non-activated γTuRC. We propose a model where generation of γTuRC with an expanded conformational range, rather than perfect symmetry, is sufficient to boost nucleation activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19570.map.gz emd_19570.map.gz | 140.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19570-v30.xml emd-19570-v30.xml emd-19570.xml emd-19570.xml | 41.7 KB 41.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19570_fsc.xml emd_19570_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_19570.png emd_19570.png | 52.7 KB | ||
| Masks |  emd_19570_msk_1.map emd_19570_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19570.cif.gz emd-19570.cif.gz | 11.5 KB | ||
| Others |  emd_19570_additional_1.map.gz emd_19570_additional_1.map.gz emd_19570_additional_2.map.gz emd_19570_additional_2.map.gz emd_19570_additional_3.map.gz emd_19570_additional_3.map.gz emd_19570_additional_4.map.gz emd_19570_additional_4.map.gz emd_19570_half_map_1.map.gz emd_19570_half_map_1.map.gz emd_19570_half_map_2.map.gz emd_19570_half_map_2.map.gz | 8.9 MB 8.9 MB 141.1 MB 165.9 MB 140.6 MB 140.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19570 http://ftp.pdbj.org/pub/emdb/structures/EMD-19570 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19570 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19570 | HTTPS FTP |
-Related structure data
| Related structure data |  8rx1MC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19570.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19570.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D Refinement map of the gTuRC-CM1dim complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.67 Å | ||||||||||||||||||||||||||||||||||||
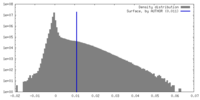
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19570_msk_1.map emd_19570_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
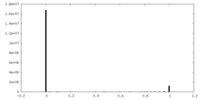
| Density Histograms |
- Sample components
Sample components
+Entire : gTuRC-CM1dim complex
+Supramolecule #1: gTuRC-CM1dim complex
+Macromolecule #1: Tubulin gamma-1 chain
+Macromolecule #2: Actin b
+Macromolecule #3: Gamma-tubulin complex component 2
+Macromolecule #4: Gamma-tubulin complex component 3
+Macromolecule #5: CM1
+Macromolecule #6: Gamma-tubulin complex component 4
+Macromolecule #7: Gamma-tubulin complex component 5
+Macromolecule #8: Gamma-tubulin complex component 6
+Macromolecule #9: Mitotic-spindle organizing protein 1
+Macromolecule #10: Mitotic-spindle organizing protein 2A
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R0.6/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 10.0 kPa |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 94094 / Average exposure time: 2.0 sec. / Average electron dose: 55.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.9 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)