+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Closed Complex I from murine brain | |||||||||
 Map data Map data | composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | respiratory chain complex / mammalian mitochondria / MEMBRANE PROTEIN / ELECTRON TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationMitochondrial protein import / response to injury involved in regulation of muscle adaptation / Protein lipoylation / Mitochondrial Fatty Acid Beta-Oxidation / Complex I biogenesis / RHOG GTPase cycle / Respiratory electron transport / protein insertion into mitochondrial inner membrane / blastocyst hatching / respiratory system process ...Mitochondrial protein import / response to injury involved in regulation of muscle adaptation / Protein lipoylation / Mitochondrial Fatty Acid Beta-Oxidation / Complex I biogenesis / RHOG GTPase cycle / Respiratory electron transport / protein insertion into mitochondrial inner membrane / blastocyst hatching / respiratory system process / psychomotor behavior / Mitochondrial protein degradation / response to light intensity / cellular response to oxygen levels / iron-sulfur cluster assembly complex / mesenchymal stem cell proliferation / reproductive system development / mitochondrial large ribosomal subunit binding / respiratory chain complex / gliogenesis / mitochondrial [2Fe-2S] assembly complex / mesenchymal stem cell differentiation / circulatory system development / negative regulation of non-canonical NF-kappaB signal transduction / adult walking behavior / cardiac muscle tissue development / positive regulation of mitochondrial membrane potential / response to hydroperoxide / neural precursor cell proliferation / [2Fe-2S] cluster assembly / oxygen sensor activity / cellular response to glucocorticoid stimulus / stem cell division / NADH dehydrogenase activity / iron-sulfur cluster assembly / dopamine metabolic process / adult behavior / NADH:ubiquinone reductase (H+-translocating) / mitochondrial ATP synthesis coupled electron transport / positive regulation of ATP biosynthetic process / mitochondrial respiratory chain complex I assembly / proton motive force-driven mitochondrial ATP synthesis / mitochondrial electron transport, NADH to ubiquinone / respiratory chain complex I / positive regulation of execution phase of apoptosis / NADH dehydrogenase (ubiquinone) activity / neuron development / quinone binding / ATP synthesis coupled electron transport / cellular response to interferon-beta / negative regulation of reactive oxygen species biosynthetic process / tricarboxylic acid cycle / extrinsic apoptotic signaling pathway / cellular response to retinoic acid / neurogenesis / Neutrophil degranulation / visual perception / reactive oxygen species metabolic process / muscle contraction / aerobic respiration / cerebellum development / regulation of mitochondrial membrane potential / respiratory electron transport chain / response to nicotine / response to cocaine / mitochondrion organization / DNA damage response, signal transduction by p53 class mediator / kidney development / monooxygenase activity / response to hydrogen peroxide / sensory perception of sound / electron transport chain / fatty acid metabolic process / circadian rhythm / brain development / mitochondrial intermembrane space / 2 iron, 2 sulfur cluster binding / mitochondrial membrane / cognition / multicellular organism growth / NAD binding / fatty acid biosynthetic process / positive regulation of protein catabolic process / cellular senescence / FMN binding / nervous system development / myelin sheath / 4 iron, 4 sulfur cluster binding / response to oxidative stress / neuron apoptotic process / response to ethanol / gene expression / in utero embryonic development / response to hypoxia / electron transfer activity / mitochondrial inner membrane / nuclear speck / nuclear body / mitochondrial matrix / response to xenobiotic stimulus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
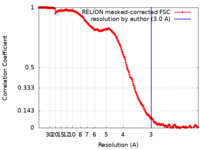
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Vercellino I / Sazanov LA | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: SCAF1 drives the compositional diversity of mammalian respirasomes. Authors: Irene Vercellino / Leonid A Sazanov /   Abstract: Supercomplexes of the respiratory chain are established constituents of the oxidative phosphorylation system, but their role in mammalian metabolism has been hotly debated. Although recent studies ...Supercomplexes of the respiratory chain are established constituents of the oxidative phosphorylation system, but their role in mammalian metabolism has been hotly debated. Although recent studies have shown that different tissues/organs are equipped with specific sets of supercomplexes, depending on their metabolic needs, the notion that supercomplexes have a role in the regulation of metabolism has been challenged. However, irrespective of the mechanistic conclusions, the composition of various high molecular weight supercomplexes remains uncertain. Here, using cryogenic electron microscopy, we demonstrate that mammalian (mouse) tissues contain three defined types of 'respirasome', supercomplexes made of CI, CIII and CIV. The stoichiometry and position of CIV differs in the three respirasomes, of which only one contains the supercomplex-associated factor SCAF1, whose involvement in respirasome formation has long been contended. Our structures confirm that the 'canonical' respirasome (the C-respirasome, CICIIICIV) does not contain SCAF1, which is instead associated to a different respirasome (the CS-respirasome), containing a second copy of CIV. We also identify an alternative respirasome (A-respirasome), with CIV bound to the 'back' of CI, instead of the 'toe'. This structural characterization of mouse mitochondrial supercomplexes allows us to hypothesize a mechanistic basis for their specific role in different metabolic conditions. #1:  Journal: Acta Crystallogr., Sect. D: Biol. Cristallogr. / Year: 2018 Journal: Acta Crystallogr., Sect. D: Biol. Cristallogr. / Year: 2018Title: Real-space refinement in PHENIX for cryo-EM and crystallography Authors: Afonine PV / Adams PD | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19145.map.gz emd_19145.map.gz | 6.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19145-v30.xml emd-19145-v30.xml emd-19145.xml emd-19145.xml | 67.2 KB 67.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19145_fsc.xml emd_19145_fsc.xml | 21.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_19145.png emd_19145.png | 100.4 KB | ||
| Filedesc metadata |  emd-19145.cif.gz emd-19145.cif.gz | 14.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19145 http://ftp.pdbj.org/pub/emdb/structures/EMD-19145 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19145 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19145 | HTTPS FTP |
-Validation report
| Summary document |  emd_19145_validation.pdf.gz emd_19145_validation.pdf.gz | 532 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19145_full_validation.pdf.gz emd_19145_full_validation.pdf.gz | 531.5 KB | Display | |
| Data in XML |  emd_19145_validation.xml.gz emd_19145_validation.xml.gz | 13.5 KB | Display | |
| Data in CIF |  emd_19145_validation.cif.gz emd_19145_validation.cif.gz | 19.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19145 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19145 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19145 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19145 | HTTPS FTP |
-Related structure data
| Related structure data |  8rgpMC  8pw5C  8pw6C  8pw7C  8rgqC  8rgrC  8rgtC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19145.map.gz / Format: CCP4 / Size: 31.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19145.map.gz / Format: CCP4 / Size: 31.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | composite map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Closed Complex I from murine brain
+Supramolecule #1: Closed Complex I from murine brain
+Macromolecule #1: NADH dehydrogenase [ubiquinone] iron-sulfur protein 7, mitochondrial
+Macromolecule #2: NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, mitochondrial
+Macromolecule #3: NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial
+Macromolecule #4: NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial
+Macromolecule #5: NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
+Macromolecule #6: NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial
+Macromolecule #7: NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial
+Macromolecule #8: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 9, mit...
+Macromolecule #9: NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, mitochondrial
+Macromolecule #10: NADH dehydrogenase [ubiquinone] iron-sulfur protein 6, mitochondrial
+Macromolecule #11: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2
+Macromolecule #12: Acyl carrier protein, mitochondrial
+Macromolecule #13: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5
+Macromolecule #14: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 6
+Macromolecule #15: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 12
+Macromolecule #16: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 7
+Macromolecule #17: NADH dehydrogenase [ubiquinone] flavoprotein 3, mitochondrial
+Macromolecule #18: NADH-ubiquinone oxidoreductase chain 3
+Macromolecule #19: NADH-ubiquinone oxidoreductase chain 1
+Macromolecule #20: NADH-ubiquinone oxidoreductase chain 6
+Macromolecule #21: NADH-ubiquinone oxidoreductase chain 4L
+Macromolecule #22: NADH-ubiquinone oxidoreductase chain 5
+Macromolecule #23: NADH-ubiquinone oxidoreductase chain 4
+Macromolecule #24: NADH-ubiquinone oxidoreductase chain 2
+Macromolecule #25: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10, mi...
+Macromolecule #26: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 8
+Macromolecule #27: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 11
+Macromolecule #28: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 13
+Macromolecule #29: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 1
+Macromolecule #30: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 3
+Macromolecule #31: NADH dehydrogenase [ubiquinone] 1 subunit C1, mitochondrial
+Macromolecule #32: NADH dehydrogenase [ubiquinone] 1 subunit C2
+Macromolecule #33: NADH dehydrogenase [ubiquinone] iron-sulfur protein 5
+Macromolecule #34: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 1
+Macromolecule #35: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 11, mit...
+Macromolecule #36: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 5, mito...
+Macromolecule #37: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6
+Macromolecule #38: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 2, mito...
+Macromolecule #39: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 3
+Macromolecule #40: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8, mito...
+Macromolecule #41: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 4
+Macromolecule #42: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9
+Macromolecule #43: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 7
+Macromolecule #44: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10
+Macromolecule #45: IRON/SULFUR CLUSTER
+Macromolecule #46: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #47: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #48: Coenzyme Q10, (2Z,6E,10Z,14E,18E,22E,26Z)-isomer
+Macromolecule #49: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #50: FLAVIN MONONUCLEOTIDE
+Macromolecule #51: POTASSIUM ION
+Macromolecule #52: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE
+Macromolecule #53: ZINC ION
+Macromolecule #54: S-[2-({N-[(2S)-2-hydroxy-3,3-dimethyl-4-(phosphonooxy)butanoyl]-b...
+Macromolecule #55: CARDIOLIPIN
+Macromolecule #56: 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #57: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.7 Component:
| ||||||||||||
| Grid | Model: Quantifoil R0.6/1 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 1 | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 10416 / Average exposure time: 4.4 sec. / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 2.6 µm / Calibrated defocus min: 0.6 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)