[English] 日本語
 Yorodumi
Yorodumi- EMDB-19002: XBB-4 Fab in complex with SARS-CoV-2 BA.2.12.1 Spike Glycoprotein -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | XBB-4 Fab in complex with SARS-CoV-2 BA.2.12.1 Spike Glycoprotein | |||||||||||||||
 Map data Map data | Sharpened locally refined cryoEM map of XBB4 fab in complex with BA.2.12.1 Spike glycoprotein focussing on RBD and fab. | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | SARS-CoV-2 / Spike / Glycoprotein / Coronavirus / antibody / Fab / XBB.1.5 / therapeutic / complex / neutralising / RBD / receptor binding domain / convalescent sera / viral protein / immune system / XBB-4 / BA.2.12.1 / omicron variant / virus / BA.2.86 | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationvirion component / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion ...virion component / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  Tequatrovirus T4 Tequatrovirus T4 | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.41 Å | |||||||||||||||
 Authors Authors | Duyvesteyn HME / Ren J / Stuart DI | |||||||||||||||
| Funding support |  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Cell Rep Med / Year: 2024 Journal: Cell Rep Med / Year: 2024Title: A structure-function analysis shows SARS-CoV-2 BA.2.86 balances antibody escape and ACE2 affinity. Authors: Chang Liu / Daming Zhou / Aiste Dijokaite-Guraliuc / Piyada Supasa / Helen M E Duyvesteyn / Helen M Ginn / Muneeswaran Selvaraj / Alexander J Mentzer / Raksha Das / Thushan I de Silva / ...Authors: Chang Liu / Daming Zhou / Aiste Dijokaite-Guraliuc / Piyada Supasa / Helen M E Duyvesteyn / Helen M Ginn / Muneeswaran Selvaraj / Alexander J Mentzer / Raksha Das / Thushan I de Silva / Thomas G Ritter / Megan Plowright / Thomas A H Newman / Lizzie Stafford / Barbara Kronsteiner / Nigel Temperton / Yuan Lui / Martin Fellermeyer / Philip Goulder / Paul Klenerman / Susanna J Dunachie / Michael I Barton / Mikhail A Kutuzov / Omer Dushek / / Elizabeth E Fry / Juthathip Mongkolsapaya / Jingshan Ren / David I Stuart / Gavin R Screaton /    Abstract: BA.2.86, a recently described sublineage of SARS-CoV-2 Omicron, contains many mutations in the spike gene. It appears to have originated from BA.2 and is distinct from the XBB variants responsible ...BA.2.86, a recently described sublineage of SARS-CoV-2 Omicron, contains many mutations in the spike gene. It appears to have originated from BA.2 and is distinct from the XBB variants responsible for many infections in 2023. The global spread and plethora of mutations in BA.2.86 has caused concern that it may possess greater immune-evasive potential, leading to a new wave of infection. Here, we examine the ability of BA.2.86 to evade the antibody response to infection using a panel of vaccinated or naturally infected sera and find that it shows marginally less immune evasion than XBB.1.5. We locate BA.2.86 in the antigenic landscape of recent variants and look at its ability to escape panels of potent monoclonal antibodies generated against contemporary SARS-CoV-2 infections. We demonstrate, and provide a structural explanation for, increased affinity of BA.2.86 to ACE2, which may increase transmissibility. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19002.map.gz emd_19002.map.gz | 322.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19002-v30.xml emd-19002-v30.xml emd-19002.xml emd-19002.xml | 23.5 KB 23.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19002_fsc.xml emd_19002_fsc.xml | 18.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_19002.png emd_19002.png | 85.5 KB | ||
| Filedesc metadata |  emd-19002.cif.gz emd-19002.cif.gz | 7.7 KB | ||
| Others |  emd_19002_half_map_1.map.gz emd_19002_half_map_1.map.gz emd_19002_half_map_2.map.gz emd_19002_half_map_2.map.gz | 289.8 MB 286.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19002 http://ftp.pdbj.org/pub/emdb/structures/EMD-19002 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19002 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19002 | HTTPS FTP |
-Validation report
| Summary document |  emd_19002_validation.pdf.gz emd_19002_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19002_full_validation.pdf.gz emd_19002_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_19002_validation.xml.gz emd_19002_validation.xml.gz | 25.1 KB | Display | |
| Data in CIF |  emd_19002_validation.cif.gz emd_19002_validation.cif.gz | 33.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19002 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19002 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19002 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19002 | HTTPS FTP |
-Related structure data
| Related structure data |  8r8kMC  8qrfC  8qrgC  8qsqC  8qtdC  8r80C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19002.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19002.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened locally refined cryoEM map of XBB4 fab in complex with BA.2.12.1 Spike glycoprotein focussing on RBD and fab. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.7303 Å | ||||||||||||||||||||||||||||||||||||
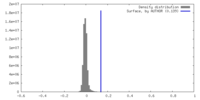
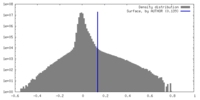
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Locally refined cryoEM half map of XBB4 fab...
| File | emd_19002_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally refined cryoEM half map of XBB4 fab in complex with BA.2.12.1 Spike glycoprotein focussing on RBD and fab. | ||||||||||||
| Projections & Slices |
| ||||||||||||
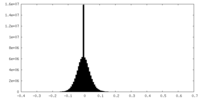
| Density Histograms |
-Half map: Locally refined cryoEM half map of XBB4 fab...
| File | emd_19002_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally refined cryoEM half map of XBB4 fab in complex with BA.2.12.1 Spike glycoprotein focussing on RBD and fab. | ||||||||||||
| Projections & Slices |
| ||||||||||||
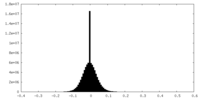
| Density Histograms |
- Sample components
Sample components
-Entire : XBB-4 Fab in complex with SARS-COV-2 BA.2.12.1 Spike Glycoprotein
| Entire | Name: XBB-4 Fab in complex with SARS-COV-2 BA.2.12.1 Spike Glycoprotein |
|---|---|
| Components |
|
-Supramolecule #1: XBB-4 Fab in complex with SARS-COV-2 BA.2.12.1 Spike Glycoprotein
| Supramolecule | Name: XBB-4 Fab in complex with SARS-COV-2 BA.2.12.1 Spike Glycoprotein type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #2: XBB-4 Fab
| Supramolecule | Name: XBB-4 Fab / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 Details: Sequence from antibody isolated from convalescent sera. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: BA.2.12.1 Spike Glycoprotein Ectodomain
| Supramolecule | Name: BA.2.12.1 Spike Glycoprotein Ectodomain / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Spike glycoprotein,Fibritin
| Macromolecule | Name: Spike glycoprotein,Fibritin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Tequatrovirus T4 Tequatrovirus T4 |
| Molecular weight | Theoretical: 142.72925 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLIT RTQSYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHAIHVSGT NGTKRFDNPV LPFNDGVYF ASTEKSNIIR GWIFGTTLDS KTQSLLIVNN ATNVVIKVCE FQFCNDPFLD VYYHKNNKSW MESEFRVYSS A NNCTFEYV ...String: MFVFLVLLPL VSSQCVNLIT RTQSYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHAIHVSGT NGTKRFDNPV LPFNDGVYF ASTEKSNIIR GWIFGTTLDS KTQSLLIVNN ATNVVIKVCE FQFCNDPFLD VYYHKNNKSW MESEFRVYSS A NNCTFEYV SQPFLMDLEG KQGNFKNLRE FVFKNIDGYF KIYSKHTPIN LGRDLPQGFS ALEPLVDLPI GINITRFQTL LA LHRSYLT PGDSSSGWTA GAAAYYVGYL QPRTFLLKYN ENGTITDAVD CALDPLSETK CTLKSFTVEK GIYQTSNFRV QPT ESIVRF PNITNLCPFD EVFNATRFAS VYAWNRKRIS NCVADYSVLY NFAPFFAFKC YGVSPTKLND LCFTNVYADS FVIR GNEVS QIAPGQTGNI ADYNYKLPDD FTGCVIAWNS NKLDSKVGGN YNYQYRLFRK SNLKPFERDI STEIYQAGNK PCNGV AGFN CYFPLRSYGF RPTYGVGHQP YRVVVLSFEL LHAPATVCGP KKSTNLVKNK CVNFNFNGLT GTGVLTESNK KFLPFQ QFG RDIADTTDAV RDPQTLEILD ITPCSFGGVS VITPGTNTSN QVAVLYQGVN CTEVPVAIHA DQLTPTWRVY STGSNVF QT RAGCLIGAEY VNNSYECDIP IGAGICASYQ TQTKSHRRAR SVASQSIIAY TMSLGAENLV AYSNNSIAIP TNFTISVT T EILPVSMTKT SVDCTMYICG DSTECSNLLL QYGSFCTQLK RALTGIAVEQ DKNTQEVFAQ VKQIYKTPPI KYFGGFNFS QILPDPSKPS KRSFIEDLLF NKVTLADAGF IKQYGDCLGD IAARDLICAQ KFNGLTVLPP LLTDEMIAQY TSALLAGTIT SGWTFGAGA ALQIPFAMQM AYRFNGIGVT QNVLYENQKL IANQFNSAIG KIQDSLSSTA SALGKLQDVV NHNAQALNTL V KQLSSKFG AISSVLNDIL SRLDKVEAEV QIDRLITGRL QSLQTYVTQQ LIRAAEIRAS ANLAATKMSE CVLGQSKRVD FC GKGYHLM SFPQSAPHGV VFLHVTYVPA QEKNFTTAPA ICHDGKAHFP REGVFVSNGT HWFVTQRNFY EPQIITTDNT FVS GNCDVV IGIVNNTVYD PLQPELDSFK EELDKYFKNH TSPDVDLGDI SGINASVVNI QKEIDRLNEV AKNLNESLID LQEL GKYEQ GSGYIPEAPR DGQAYVRKDG EWVLLSTFLG RSLEVLFQGP GHHHHHHHHG SAWSHPQFEK GGGSGGGSGG SAWSH PQFE K UniProtKB: Spike glycoprotein, Fibritin |
-Macromolecule #2: XBB-4 Fab Heavy chain
| Macromolecule | Name: XBB-4 Fab Heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.75566 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVQSGGG LVKPGGSLRL SCAASGFSFT NAWMNWVRQA PGKGLEWVGR IKSKADGGTT DYAAPVKGKF TISRDDSKNT LYLQMNSLK TEDTAIYYCT SDVYDFSTGF GQRDDFDYWG QGTLVTVSSA STKGPSVFPL APSSKSTSGG TAALGCLVKD Y FPEPVTVS ...String: EVQLVQSGGG LVKPGGSLRL SCAASGFSFT NAWMNWVRQA PGKGLEWVGR IKSKADGGTT DYAAPVKGKF TISRDDSKNT LYLQMNSLK TEDTAIYYCT SDVYDFSTGF GQRDDFDYWG QGTLVTVSSA STKGPSVFPL APSSKSTSGG TAALGCLVKD Y FPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP KSCDK |
-Macromolecule #3: XBB-4 Fab Light chain
| Macromolecule | Name: XBB-4 Fab Light chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.18575 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DIVMTQSPSS LSASVGDRVT ITCRASQSIS YFLNWYQQKP GKAPKLLISA ASSLQSGVPS RFSGSGSGTD FTLTISSLQP EDFATYYCQ QSYSSLITFG GGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS ...String: DIVMTQSPSS LSASVGDRVT ITCRASQSIS YFLNWYQQKP GKAPKLLISA ASSLQSGVPS RFSGSGSGTD FTLTISSLQP EDFATYYCQ QSYSSLITFG GGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS KDSTYSLSST LTLSKADYEK HKVYACEVTH QGLSSPVTKS FNRGEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.5 K / Instrument: FEI VITROBOT MARK IV |
| Details | Spike ectodomain incubated with XBB-4 Fab in 2-fold molecular excess of sites (assuming three per trimeric spike unit). |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 165000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)