[English] 日本語
 Yorodumi
Yorodumi- EMDB-18156: Combined map of Candida albicans 80S ribosome in complex with cep... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Combined map of Candida albicans 80S ribosome in complex with cephaeline | |||||||||
 Map data Map data | Composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ribosome / Candida albicans / cephaeline | |||||||||
| Function / homology |  Function and homology information Function and homology informationyeast-form cell wall / hyphal cell wall / preribosome / positive regulation of translational fidelity / pre-mRNA 5'-splice site binding / nonfunctional rRNA decay / preribosome, small subunit precursor / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / translational elongation ...yeast-form cell wall / hyphal cell wall / preribosome / positive regulation of translational fidelity / pre-mRNA 5'-splice site binding / nonfunctional rRNA decay / preribosome, small subunit precursor / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / translational elongation / 90S preribosome / ribosomal subunit export from nucleus / protein-RNA complex assembly / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / ribosomal small subunit export from nucleus / DNA-(apurinic or apyrimidinic site) endonuclease activity / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA / small-subunit processome / maintenance of translational fidelity / rRNA processing / large ribosomal subunit / ribosome biogenesis / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / nucleolus / cell surface / RNA binding / zinc ion binding / metal ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Candida albicans (yeast) Candida albicans (yeast) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.45 Å | |||||||||
 Authors Authors | Kolosova O / Zgadzay Y / Stetsenko A / Atamas A / Jenner L / Guskov A / Yusupov M | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Biopolym Cell / Year: 2023 Journal: Biopolym Cell / Year: 2023Title: Structural characterization of cephaeline binding to the eukaryotic ribosome using Cryo-Electron Microscopy Authors: Kolosova O / Zgadzay Y / Stetsenko A / Atamas A / Wu C / Jenner L / Sachs SM / Guskov A / Yusupov M | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18156.map.gz emd_18156.map.gz | 401.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18156-v30.xml emd-18156-v30.xml emd-18156.xml emd-18156.xml | 8.2 KB 8.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_18156.png emd_18156.png | 130.3 KB | ||
| Filedesc metadata |  emd-18156.cif.gz emd-18156.cif.gz | 3.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18156 http://ftp.pdbj.org/pub/emdb/structures/EMD-18156 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18156 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18156 | HTTPS FTP |
-Related structure data
| Related structure data |  8q5iMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18156.map.gz / Format: CCP4 / Size: 506 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18156.map.gz / Format: CCP4 / Size: 506 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.836 Å | ||||||||||||||||||||||||||||||||||||
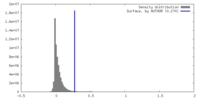
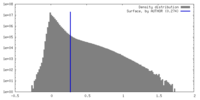
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : The Candida albicans ribosome in complew with cephaeline
| Entire | Name: The Candida albicans ribosome in complew with cephaeline |
|---|---|
| Components |
|
-Supramolecule #1: The Candida albicans ribosome in complew with cephaeline
| Supramolecule | Name: The Candida albicans ribosome in complew with cephaeline type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Candida albicans (yeast) Candida albicans (yeast) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.9 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.45 Å / Resolution method: OTHER / Number images used: 254047 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)