+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Befiradol-bound serotonin 5-HT1A receptor - Gi Protein Complex | ||||||||||||
 Map data Map data | sharpened and filtered map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway ...regulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway / neurotransmitter receptor activity / serotonin metabolic process / serotonin binding / gamma-aminobutyric acid signaling pathway / exploration behavior / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / regulation of vasoconstriction / behavioral fear response / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to peptide hormone / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / GDP binding / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / chemical synaptic transmission / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / G protein-coupled receptor signaling pathway / cell division / lysosomal membrane / GTPase activity / positive regulation of cell population proliferation Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
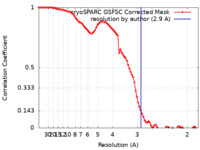
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | ||||||||||||
 Authors Authors | Schneider J / Gmeiner P / Hove TT / Rasmussen T / Boettcher B | ||||||||||||
| Funding support | European Union,  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2025 Journal: Sci Adv / Year: 2025Title: Discovery of a functionally selective serotonin receptor (5-HTR) agonist for the treatment of pain. Authors: Annika Ullrich / Johannes Schneider / João M Braz / Eduard Neu / Nico Staffen / Markus Stanek / Jana Bláhová / Tamsanqa Hove / Tamara Albert / Anni Allikalt / Stefan Löber / Karnika ...Authors: Annika Ullrich / Johannes Schneider / João M Braz / Eduard Neu / Nico Staffen / Markus Stanek / Jana Bláhová / Tamsanqa Hove / Tamara Albert / Anni Allikalt / Stefan Löber / Karnika Bhardwaj / Sian Rodriguez-Rosado / Elissa Fink / Tim Rasmussen / Harald Hübner / Asuka Inoue / Brian K Shoichet / Allan I Basbaum / Bettina Böttcher / Dorothee Weikert / Peter Gmeiner /    Abstract: The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, ...The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, we evolved ST171, a bitopic 5-HTR agonist that revealed highly potent and functionally selective G signaling without G activation and marginal β-arrestin recruitment. ST171 is effective in acute and chronic pain models. Cryo-electron microscopy structures of ST171 bound to 5-HTR in complex with the G protein compared to the canonical agonist befiradol bound to complexes of 5-HTR with G or G revealed that the ligands occupy different exo-sites. The individual binding poses are associated with ligand-specific receptor conformations that were further studied by molecular dynamics simulations, allowing us to better understand ligand bias, a phenomenon that may be crucial to the discovery of more effective and safe G protein-coupled receptor drugs. #1:  Journal: Biorxiv / Year: 2023 Journal: Biorxiv / Year: 2023Title: Discovery of a functionally selective serotonin 5-HT1A receptor agonist for the treatment of pain Authors: Ullrich A / Schneider J / Braz JM / Neu E / Staffen N / Stanek M / Blahova J / Hove T / Albert T / Allikalt A / Lober S / Bhardwaj K / Rodriguez-Rosado S / Fink E / Rasmussen T / Hubner H / ...Authors: Ullrich A / Schneider J / Braz JM / Neu E / Staffen N / Stanek M / Blahova J / Hove T / Albert T / Allikalt A / Lober S / Bhardwaj K / Rodriguez-Rosado S / Fink E / Rasmussen T / Hubner H / Inoue A / Shoichet BK / Basbaum AJ / Bottcher B / Weikert D / Gmeiner P | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17747.map.gz emd_17747.map.gz | 58.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17747-v30.xml emd-17747-v30.xml emd-17747.xml emd-17747.xml | 29.5 KB 29.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17747_fsc.xml emd_17747_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_17747.png emd_17747.png | 141.6 KB | ||
| Filedesc metadata |  emd-17747.cif.gz emd-17747.cif.gz | 8.3 KB | ||
| Others |  emd_17747_half_map_1.map.gz emd_17747_half_map_1.map.gz emd_17747_half_map_2.map.gz emd_17747_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17747 http://ftp.pdbj.org/pub/emdb/structures/EMD-17747 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17747 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17747 | HTTPS FTP |
-Related structure data
| Related structure data |  8pkmMC  8pjkC  9gl2C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17747.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17747.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened and filtered map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.91375 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_17747_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17747_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : befiradol-bound Serotonin 5-HT1A receptor - Gi Protein Complex
| Entire | Name: befiradol-bound Serotonin 5-HT1A receptor - Gi Protein Complex |
|---|---|
| Components |
|
-Supramolecule #1: befiradol-bound Serotonin 5-HT1A receptor - Gi Protein Complex
| Supramolecule | Name: befiradol-bound Serotonin 5-HT1A receptor - Gi Protein Complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: Complex of human serotonin 5-HT1A receptor and Gi Protein bound to the agonist befiradol |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.414047 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.810352 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHHHG SSGSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAKI YAMHWGTDSR LLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK TREGNVRVSR ELAGHTGYLS C CRFLDDNQ ...String: MHHHHHHHHG SSGSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAKI YAMHWGTDSR LLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK TREGNVRVSR ELAGHTGYLS C CRFLDDNQ IVTSSGDTTC ALWDIETGQQ TTTFTGHTGD VMSLSLAPDT RLFVSGACDA SAKLWDVREG MCRQTFTGHE SD INAICFF PNGNAFATGS DDATCRLFDL RADQELMTYS HDNIICGITS VSFSKSGRLL LAGYDDFNCN VWDALKADRA GVL AGHDNR VSCLGVTDDG MAVATGSWDS FLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: 5-hydroxytryptamine receptor 1A
| Macromolecule | Name: 5-hydroxytryptamine receptor 1A / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.431578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDAAAAHHH HHHHHHHENL YFQGMDVLSP GQGNNTTSPP APFETGGNTT GISDVTVSYQ VITSLLLGT LIFCAVLGNA CVVAAIALER SLQNVANYLI GSLAVTDLMV SVLVLPMAAL YQVLNKWTLG QVTCDLFIAL D VLCCTSSI ...String: MKTIIALSYI FCLVFADYKD DDDAAAAHHH HHHHHHHENL YFQGMDVLSP GQGNNTTSPP APFETGGNTT GISDVTVSYQ VITSLLLGT LIFCAVLGNA CVVAAIALER SLQNVANYLI GSLAVTDLMV SVLVLPMAAL YQVLNKWTLG QVTCDLFIAL D VLCCTSSI WHLCAIALDR YWAITDPIDY VNKRTPRRAA ALISLTWLIG FLISIPPMLG WRTPEDRSDP DACTISKDHG YT IYSTFGA FYIPLLLMLV LYGRIFRAAR FRIRKTVKKV EKTGADTRHG ASPAPQPKKS VNGESGSRNW RLGVESKAGG ALC ANGAVR QGDDGAALEV IEVHRVGNSK EHLPLPSEAG PTPCAPASFE RKNERNAEAK RKMALARERK TVKTLGIIMG TFIL CWLPF FIVALVLPFC ESSCHMPTLL GAIINWLGYS NSLLNPVIYA YFNKDFQNAF KKIIKCKFCR Q UniProtKB: 5-hydroxytryptamine receptor 1A |
-Macromolecule #5: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 5 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #6: (2R)-1-(heptadecanoyloxy)-3-{[(R)-hydroxy{[(1R,2R,3R,4R,5S,6R)-2,...
| Macromolecule | Name: (2R)-1-(heptadecanoyloxy)-3-{[(R)-hydroxy{[(1R,2R,3R,4R,5S,6R)-2,3,5,6-tetrahydroxy-4-(phosphonooxy)cyclohexyl]oxy}phosphoryl]oxy}propan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate type: ligand / ID: 6 / Number of copies: 1 / Formula: T7M |
|---|---|
| Molecular weight | Theoretical: 953.081 Da |
| Chemical component information | 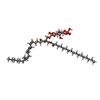 ChemComp-T7M: |
-Macromolecule #7: (3-chloranyl-4-fluoranyl-phenyl)-[4-fluoranyl-4-[[(5-methylpyridi...
| Macromolecule | Name: (3-chloranyl-4-fluoranyl-phenyl)-[4-fluoranyl-4-[[(5-methylpyridin-2-yl)methylamino]methyl]piperidin-1-yl]methanone type: ligand / ID: 7 / Number of copies: 1 / Formula: ZKV |
|---|---|
| Molecular weight | Theoretical: 393.858 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.9 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 100 mM NaCl, 20 mM HEPES, 0.002% LMNG, 0.0004 CHS | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | This sample was monodisperse with a few impurities through dimeric complexes. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 11578 / Average exposure time: 5.8 sec. / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8pkm: |
 Movie
Movie Controller
Controller


































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)