+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-1748 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Asymmetric organization of connexin26 gap junction channels | |||||||||
 マップデータ マップデータ | Three dimensional structure of Cx26M34A at 6 angstrom resolution | |||||||||
 試料 試料 |
| |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Transport of connexons to the plasma membrane / gap junction-mediated intercellular transport / gap junction channel activity involved in cell communication by electrical coupling / Oligomerization of connexins into connexons / Transport of connexins along the secretory pathway / gap junction assembly / connexin complex / gap junction / gap junction channel activity / Gap junction assembly ...Transport of connexons to the plasma membrane / gap junction-mediated intercellular transport / gap junction channel activity involved in cell communication by electrical coupling / Oligomerization of connexins into connexons / Transport of connexins along the secretory pathway / gap junction assembly / connexin complex / gap junction / gap junction channel activity / Gap junction assembly / endoplasmic reticulum-Golgi intermediate compartment / sensory perception of sound / transmembrane transport / cell-cell signaling / calcium ion binding / identical protein binding / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 電子線結晶学 / クライオ電子顕微鏡法 / ネガティブ染色法 / 解像度: 6.0 Å | |||||||||
 データ登録者 データ登録者 | Oshima A / Tani K / Toloue MM / Hiroaki Y / Smock A / Inukai S / Cone A / Nicholson BJ / Sosinsky GE / Fujiyoshi Y | |||||||||
 引用 引用 |  ジャーナル: J Mol Biol / 年: 2011 ジャーナル: J Mol Biol / 年: 2011タイトル: Asymmetric configurations and N-terminal rearrangements in connexin26 gap junction channels. 著者: Atsunori Oshima / Kazutoshi Tani / Masoud M Toloue / Yoko Hiroaki / Amy Smock / Sayaka Inukai / Angela Cone / Bruce J Nicholson / Gina E Sosinsky / Yoshinori Fujiyoshi /  要旨: Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the N terminus as a key component in gating, and possibly assembly. Here, we ...Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the N terminus as a key component in gating, and possibly assembly. Here, we present electron crystallographic structures of a mutant human connexin26 (Cx26M34A) and an N-terminal deletion of this mutant (Cx26M34Adel2-7) at 6-Å and 10-Å resolutions, respectively. The three-dimensional map of Cx26M34A was improved by data from 60° tilt images and revealed a breakdown of the hexagonal symmetry in a connexin hemichannel, particularly in the cytoplasmic domain regions at the ends of the transmembrane helices. The Cx26M34A structure contained an asymmetric density in the channel vestibule ("plug") that was decreased in the Cx26M34Adel2-7 structure, indicating that the N terminus significantly contributes to form this plug feature. Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the recently published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_1748.map.gz emd_1748.map.gz | 1.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-1748-v30.xml emd-1748-v30.xml emd-1748.xml emd-1748.xml | 14.7 KB 14.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  1748.png 1748.png | 443 KB | ||
| マスクデータ |  emd_1748_msk_1.map emd_1748_msk_1.map | 1.9 MB |  マスクマップ マスクマップ | |
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1748 http://ftp.pdbj.org/pub/emdb/structures/EMD-1748 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1748 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1748 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  3iz1MC  1749C  3iz2C M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_1748.map.gz / 形式: CCP4 / 大きさ: 1.8 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_1748.map.gz / 形式: CCP4 / 大きさ: 1.8 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Three dimensional structure of Cx26M34A at 6 angstrom resolution | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 これらの図は立方格子座標系で作成されたものです | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X: 1.98 Å / Y: 2.01 Å / Z: 2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
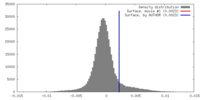
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-セグメンテーションマップ: Three dimensional structure of Cx26M34A at 6 angstrom resolution
| 注釈 | Three dimensional structure of Cx26M34A at 6 angstrom resolution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ファイル |  emd_1748_msk_1.map emd_1748_msk_1.map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
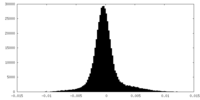
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Human connexin26 gap junction channel
| 全体 | 名称: Human connexin26 gap junction channel |
|---|---|
| 要素 |
|
-超分子 #1000: Human connexin26 gap junction channel
| 超分子 | 名称: Human connexin26 gap junction channel / タイプ: sample / ID: 1000 / 集合状態: Dodecameric / Number unique components: 1 |
|---|---|
| 分子量 | 理論値: 300 KDa |
-超分子 #1: Gap junction
| 超分子 | 名称: Gap junction / タイプ: organelle_or_cellular_component / ID: 1 / Name.synonym: Connexin / 集合状態: Dodecamer / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) / 別称: Human Homo sapiens (ヒト) / 別称: Human |
| 組換発現 | 生物種:  組換プラスミド: pBlueBac4.5 |
-実験情報
-構造解析
| 手法 | ネガティブ染色法, クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 電子線結晶学 |
| 試料の集合状態 | 2D array |
- 試料調製
試料調製
| 濃度 | 2 mg/mL |
|---|---|
| 緩衝液 | pH: 5.8 詳細: 10 mM MES, pH 5.8, 100 mM NaCl, 50 mM MgCl2, 5 mM CaCl2, 2 mM DTT, 100 uM carbenoxolone, 0.005% NaN3, 1% glycerol |
| 染色 | タイプ: NEGATIVE / 詳細: Embedded in ice with 10% trehalose |
| グリッド | 詳細: Molybdenum grid |
| 凍結 | 凍結剤: NITROGEN / チャンバー内温度: 100 K / 装置: LEICA KF80 / 詳細: Vitrification instrument: Reichert KF-80 手法: The grids were blotted with filter paper and fast frozen into liquid nitrogen |
| 詳細 | Crystals grown in three lipid bilayers |
| 結晶化 | 詳細: Crystals grown in three lipid bilayers |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | JEOL KYOTO-3000SFF |
|---|---|
| 温度 | 最低: 4 K / 最高: 4 K / 平均: 4 K |
| アライメント法 | Legacy - 非点収差: Objective astigmatism was corrected using a quadrupole stigmator at 250,000 magnification |
| 撮影 | カテゴリ: CCD / フィルム・検出器のモデル: KODAK SO-163 FILM / デジタル化 - サンプリング間隔: 7 µm / 実像数: 179 / 平均電子線量: 25 e/Å2 |
| Tilt angle min | 0 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 39000 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 1.6 mm / 最大 デフォーカス(公称値): 3.939 µm / 最小 デフォーカス(公称値): 0.458 µm / 倍率(公称値): 40000 |
| 試料ステージ | 試料ホルダー: Top entry helium cooled cryo stage / 試料ホルダーモデル: JEOL / Tilt angle max: 60 / Tilt series - Axis1 - Min angle: 0 ° / Tilt series - Axis1 - Max angle: 60 ° |
- 画像解析
画像解析
| 最終 再構成 | アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 6.0 Å / 解像度の算出法: OTHER / ソフトウェア - 名称: MRC |
|---|---|
| 結晶パラメータ | 単位格子 - A: 112.4 Å / 単位格子 - B: 111.2 Å / 単位格子 - C: 300 Å / 単位格子 - γ: 90 ° / 単位格子 - α: 90 ° / 単位格子 - β: 90 ° / 面群: P 2 21 21 |
| CTF補正 | 詳細: Each image |
-原子モデル構築 1
| 初期モデル | PDB ID: Chain - Chain ID: A |
|---|---|
| ソフトウェア | 名称: Situs |
| 詳細 | PDBEntryID_givenInChain. Protocol: Rigid body. The chain containing the amino acids from 18 to 217 corresponding to a connexin monomer was initially fitted manually into each subunit in the cryo-EM structure using program O. |
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT / 当てはまり具合の基準: Linear cross correlation |
| 得られたモデル |  PDB-3iz1: |
 ムービー
ムービー コントローラー
コントローラー




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)