+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1748 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric organization of connexin26 gap junction channels | |||||||||
 Map data Map data | Three dimensional structure of Cx26M34A at 6 angstrom resolution | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationTransport of connexons to the plasma membrane / gap junction channel activity involved in cell communication by electrical coupling / gap junction-mediated intercellular transport / Oligomerization of connexins into connexons / Transport of connexins along the secretory pathway / gap junction assembly / connexin complex / gap junction / Gap junction assembly / gap junction channel activity ...Transport of connexons to the plasma membrane / gap junction channel activity involved in cell communication by electrical coupling / gap junction-mediated intercellular transport / Oligomerization of connexins into connexons / Transport of connexins along the secretory pathway / gap junction assembly / connexin complex / gap junction / Gap junction assembly / gap junction channel activity / endoplasmic reticulum-Golgi intermediate compartment / sensory perception of sound / transmembrane transport / cell-cell signaling / calcium ion binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | electron crystallography / cryo EM / negative staining / Resolution: 6.0 Å | |||||||||
 Authors Authors | Oshima A / Tani K / Toloue MM / Hiroaki Y / Smock A / Inukai S / Cone A / Nicholson BJ / Sosinsky GE / Fujiyoshi Y | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2011 Journal: J Mol Biol / Year: 2011Title: Asymmetric configurations and N-terminal rearrangements in connexin26 gap junction channels. Authors: Atsunori Oshima / Kazutoshi Tani / Masoud M Toloue / Yoko Hiroaki / Amy Smock / Sayaka Inukai / Angela Cone / Bruce J Nicholson / Gina E Sosinsky / Yoshinori Fujiyoshi /  Abstract: Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the N terminus as a key component in gating, and possibly assembly. Here, we ...Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the N terminus as a key component in gating, and possibly assembly. Here, we present electron crystallographic structures of a mutant human connexin26 (Cx26M34A) and an N-terminal deletion of this mutant (Cx26M34Adel2-7) at 6-Å and 10-Å resolutions, respectively. The three-dimensional map of Cx26M34A was improved by data from 60° tilt images and revealed a breakdown of the hexagonal symmetry in a connexin hemichannel, particularly in the cytoplasmic domain regions at the ends of the transmembrane helices. The Cx26M34A structure contained an asymmetric density in the channel vestibule ("plug") that was decreased in the Cx26M34Adel2-7 structure, indicating that the N terminus significantly contributes to form this plug feature. Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the recently published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1748.map.gz emd_1748.map.gz | 1.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1748-v30.xml emd-1748-v30.xml emd-1748.xml emd-1748.xml | 14.7 KB 14.7 KB | Display Display |  EMDB header EMDB header |
| Images |  1748.png 1748.png | 443 KB | ||
| Masks |  emd_1748_msk_1.map emd_1748_msk_1.map | 1.9 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1748 http://ftp.pdbj.org/pub/emdb/structures/EMD-1748 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1748 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1748 | HTTPS FTP |
-Related structure data
| Related structure data |  3iz1MC  1749C  3iz2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1748.map.gz / Format: CCP4 / Size: 1.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1748.map.gz / Format: CCP4 / Size: 1.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Three dimensional structure of Cx26M34A at 6 angstrom resolution | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X: 1.98 Å / Y: 2.01 Å / Z: 2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
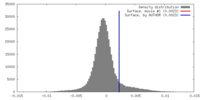
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: Three dimensional structure of Cx26M34A at 6 angstrom resolution
| Annotation | Three dimensional structure of Cx26M34A at 6 angstrom resolution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_1748_msk_1.map emd_1748_msk_1.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
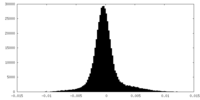
| Density Histograms |
- Sample components
Sample components
-Entire : Human connexin26 gap junction channel
| Entire | Name: Human connexin26 gap junction channel |
|---|---|
| Components |
|
-Supramolecule #1000: Human connexin26 gap junction channel
| Supramolecule | Name: Human connexin26 gap junction channel / type: sample / ID: 1000 / Oligomeric state: Dodecameric / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 300 KDa |
-Supramolecule #1: Gap junction
| Supramolecule | Name: Gap junction / type: organelle_or_cellular_component / ID: 1 / Name.synonym: Connexin / Oligomeric state: Dodecamer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | electron crystallography |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 5.8 Details: 10 mM MES, pH 5.8, 100 mM NaCl, 50 mM MgCl2, 5 mM CaCl2, 2 mM DTT, 100 uM carbenoxolone, 0.005% NaN3, 1% glycerol |
| Staining | Type: NEGATIVE / Details: Embedded in ice with 10% trehalose |
| Grid | Details: Molybdenum grid |
| Vitrification | Cryogen name: NITROGEN / Chamber temperature: 100 K / Instrument: LEICA KF80 / Details: Vitrification instrument: Reichert KF-80 Method: The grids were blotted with filter paper and fast frozen into liquid nitrogen |
| Details | Crystals grown in three lipid bilayers |
| Crystal formation | Details: Crystals grown in three lipid bilayers |
- Electron microscopy
Electron microscopy
| Microscope | JEOL KYOTO-3000SFF |
|---|---|
| Temperature | Min: 4 K / Max: 4 K / Average: 4 K |
| Alignment procedure | Legacy - Astigmatism: Objective astigmatism was corrected using a quadrupole stigmator at 250,000 magnification |
| Image recording | Category: CCD / Film or detector model: KODAK SO-163 FILM / Digitization - Sampling interval: 7 µm / Number real images: 179 / Average electron dose: 25 e/Å2 |
| Tilt angle min | 0 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 39000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 1.6 mm / Nominal defocus max: 3.939 µm / Nominal defocus min: 0.458 µm / Nominal magnification: 40000 |
| Sample stage | Specimen holder: Top entry helium cooled cryo stage / Specimen holder model: JEOL / Tilt angle max: 60 / Tilt series - Axis1 - Min angle: 0 ° / Tilt series - Axis1 - Max angle: 60 ° |
- Image processing
Image processing
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 6.0 Å / Resolution method: OTHER / Software - Name: MRC |
|---|---|
| Crystal parameters | Unit cell - A: 112.4 Å / Unit cell - B: 111.2 Å / Unit cell - C: 300 Å / Unit cell - γ: 90 ° / Unit cell - α: 90 ° / Unit cell - β: 90 ° / Plane group: P 2 21 21 |
| CTF correction | Details: Each image |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name: Situs |
| Details | PDBEntryID_givenInChain. Protocol: Rigid body. The chain containing the amino acids from 18 to 217 corresponding to a connexin monomer was initially fitted manually into each subunit in the cryo-EM structure using program O. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Linear cross correlation |
| Output model |  PDB-3iz1: |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)