[English] 日本語
 Yorodumi
Yorodumi- EMDB-17147: free 50S with additional S4-LSU in native untreated Mycoplasma pn... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | free 50S with additional S4-LSU in native untreated Mycoplasma pneumoniae cells | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | In situ / Ribosome heterogeneity / S4 / cryo-ET / RIBOSOME | |||||||||
| Biological species |  Mycoplasmoides pneumoniae M129 (bacteria) Mycoplasmoides pneumoniae M129 (bacteria) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 7.9 Å | |||||||||
 Authors Authors | Xue L / Mahamid J | |||||||||
| Funding support | European Union,  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Visualizing translation dynamics at atomic detail inside a bacterial cell. Authors: Liang Xue / Swantje Lenz / Maria Zimmermann-Kogadeeva / Dimitry Tegunov / Patrick Cramer / Peer Bork / Juri Rappsilber / Julia Mahamid /    Abstract: Translation is the fundamental process of protein synthesis and is catalysed by the ribosome in all living cells. Here we use advances in cryo-electron tomography and sub-tomogram analysis to ...Translation is the fundamental process of protein synthesis and is catalysed by the ribosome in all living cells. Here we use advances in cryo-electron tomography and sub-tomogram analysis to visualize the structural dynamics of translation inside the bacterium Mycoplasma pneumoniae. To interpret the functional states in detail, we first obtain a high-resolution in-cell average map of all translating ribosomes and build an atomic model for the M. pneumoniae ribosome that reveals distinct extensions of ribosomal proteins. Classification then resolves 13 ribosome states that differ in their conformation and composition. These recapitulate major states that were previously resolved in vitro, and reflect intermediates during active translation. On the basis of these states, we animate translation elongation inside native cells and show how antibiotics reshape the cellular translation landscapes. During translation elongation, ribosomes often assemble in defined three-dimensional arrangements to form polysomes. By mapping the intracellular organization of translating ribosomes, we show that their association into polysomes involves a local coordination mechanism that is mediated by the ribosomal protein L9. We propose that an extended conformation of L9 within polysomes mitigates collisions to facilitate translation fidelity. Our work thus demonstrates the feasibility of visualizing molecular processes at atomic detail inside cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17147.map.gz emd_17147.map.gz | 2.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17147-v30.xml emd-17147-v30.xml emd-17147.xml emd-17147.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17147_fsc.xml emd_17147_fsc.xml | 7.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_17147.png emd_17147.png | 70.4 KB | ||
| Masks |  emd_17147_msk_1.map emd_17147_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17147.cif.gz emd-17147.cif.gz | 5.4 KB | ||
| Others |  emd_17147_half_map_1.map.gz emd_17147_half_map_1.map.gz emd_17147_half_map_2.map.gz emd_17147_half_map_2.map.gz | 23.4 MB 23.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17147 http://ftp.pdbj.org/pub/emdb/structures/EMD-17147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17147 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17147.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17147.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.4 Å | ||||||||||||||||||||||||||||||||||||
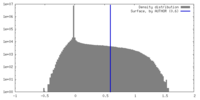
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17147_msk_1.map emd_17147_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17147_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17147_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Native untreated Mycoplasma pneumoniae M129 cells
| Entire | Name: Native untreated Mycoplasma pneumoniae M129 cells |
|---|---|
| Components |
|
-Supramolecule #1: Native untreated Mycoplasma pneumoniae M129 cells
| Supramolecule | Name: Native untreated Mycoplasma pneumoniae M129 cells / type: cell / ID: 1 / Parent: 0 Details: cryo-electron tomograms of untreated Mycoplasma pneumoniae cells growing in the fast-growing phase |
|---|---|
| Source (natural) | Organism:  Mycoplasmoides pneumoniae M129 (bacteria) Mycoplasmoides pneumoniae M129 (bacteria) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Details: The modified Hayflick medium: 14.7g/L Difco PPLO(Becton Dickinson), 20% (v/v) Gibco horse serum(New Zealand origin), 100 mM HEPES-Na; pH 7.4, 1% (w/w) glucose, 0.002% (w/w) phenol red, 1000 U/mL penicillin G. |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE-PROPANE Details: Back-side blotting for 2-3 second before plunging using a manual plunger without an environmental chamber.. |
| Details | Mycoplasma pneumoniae M129 cells grown on gold Quantifoil grids at 37 degrees Celsius before plunge freezing. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 3.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.75 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)