[English] 日本語
 Yorodumi
Yorodumi- EMDB-16884: Core-binding domain of fungal E3-binding domain bound to the nati... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Core-binding domain of fungal E3-binding domain bound to the native pyruvate dehydrogenase E2 core | |||||||||
 Map data Map data | N. crassa native PDC core with E3BP interior trimer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / metabolism / mitochondria / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationdihydrolipoyllysine-residue acetyltransferase / dihydrolipoyllysine-residue acetyltransferase activity / pyruvate decarboxylation to acetyl-CoA / pyruvate dehydrogenase complex / acyltransferase activity / mitochondrial matrix / structural molecule activity / mitochondrion Similarity search - Function | |||||||||
| Biological species |  Neurospora crassa (fungus) Neurospora crassa (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Forsberg BO | |||||||||
| Funding support |  Sweden, 1 items Sweden, 1 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: The structure and evolutionary diversity of the fungal E3-binding protein. Authors: Bjoern O Forsberg /   Abstract: The pyruvate dehydrogenase complex (PDC) is a central metabolic enzyme in all living cells composed majorly of E1, E2, and E3. Tight coupling of their reactions makes each component essential, so ...The pyruvate dehydrogenase complex (PDC) is a central metabolic enzyme in all living cells composed majorly of E1, E2, and E3. Tight coupling of their reactions makes each component essential, so that any loss impacts oxidative metabolism pathologically. E3 retention is mediated by the E3-binding protein (E3BP), which is here resolved within the PDC core from N.crassa, resolved to 3.2Å. Fungal and mammalian E3BP are shown to be orthologs, arguing E3BP as a broadly eukaryotic gene. Fungal E3BP architectures predicted from sequence data and computational models further bridge the evolutionary distance between N.crassa and humans, and suggest discriminants for E3-specificity. This is confirmed by similarities in their respective E3-binding domains, where an interaction previously not described is also predicted. This provides evolutionary parallels for a crucial interaction human metabolism, an interaction specific to fungi that can be targeted, and an example of protein evolution following gene neofunctionalization. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16884.map.gz emd_16884.map.gz | 19.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16884-v30.xml emd-16884-v30.xml emd-16884.xml emd-16884.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16884.png emd_16884.png | 183.6 KB | ||
| Filedesc metadata |  emd-16884.cif.gz emd-16884.cif.gz | 5.6 KB | ||
| Others |  emd_16884_half_map_1.map.gz emd_16884_half_map_1.map.gz emd_16884_half_map_2.map.gz emd_16884_half_map_2.map.gz | 96.7 MB 96.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16884 http://ftp.pdbj.org/pub/emdb/structures/EMD-16884 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16884 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16884 | HTTPS FTP |
-Related structure data
| Related structure data |  8ohsMC  7r5mC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16884.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16884.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | N. crassa native PDC core with E3BP interior trimer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.25 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_16884_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
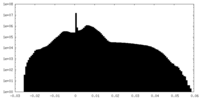
| Projections & Slices |
| ||||||||||||
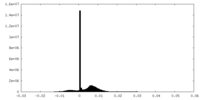
| Density Histograms |
-Half map: #2
| File | emd_16884_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
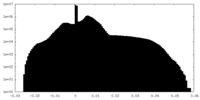
| Projections & Slices |
| ||||||||||||
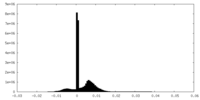
| Density Histograms |
- Sample components
Sample components
-Entire : Native pyruvate dehydrogenase complex
| Entire | Name: Native pyruvate dehydrogenase complex |
|---|---|
| Components |
|
-Supramolecule #1: Native pyruvate dehydrogenase complex
| Supramolecule | Name: Native pyruvate dehydrogenase complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
-Macromolecule #1: Dihydrolipoyllysine-residue acetyltransferase component of pyruva...
| Macromolecule | Name: Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, mitochondrial type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue acetyltransferase |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 48.677395 KDa |
| Sequence | String: MIVPVLSRQA LRHASVARVA LPSLTRWYAS YPPHTVVKMP ALSPTMTSGG IGAWQKKPGD KIEPGEVLVE IETDKAQMDF EFQEEGVLA KILKDSGEKD VAVGNPIAIL VEEGTDVNAF KDFTLKDAGG ETSPAVPKDE PKNESTASAP TPAPTPAPEP E NTSFTGRF ...String: MIVPVLSRQA LRHASVARVA LPSLTRWYAS YPPHTVVKMP ALSPTMTSGG IGAWQKKPGD KIEPGEVLVE IETDKAQMDF EFQEEGVLA KILKDSGEKD VAVGNPIAIL VEEGTDVNAF KDFTLKDAGG ETSPAVPKDE PKNESTASAP TPAPTPAPEP E NTSFTGRF QTALEREPNA LPAAKRLARE KGIDLRNVKG SGPGGKITEE DVKKALASAP AAGAAAAAYT DVPISGMRKT IA ARLKESV TENPHFFVST NLSVSKLLKL RQALNSSADG RYKLSVNDFL IKAMGIASKR VPTVNSSWRD GVIRQFETVD VSV AVATPN GLITPIVKGV EGKGLESISA AVKELAKKAR DGKLKPEEYQ GGSISISNMG MNPAVQSFTA IINPPQAAIL AVGA PQKVA VPVENEDGTT GVSWDEQIIV TASFDHKVVD GAVGAEWIRE LKKVIENPLE LLL UniProtKB: Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, mitochondrial |
-Macromolecule #2: Pyruvate dehydrogenase X component
| Macromolecule | Name: Pyruvate dehydrogenase X component / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 44.880066 KDa |
| Sequence | String: MASLTAACRI SARMAGRSVR GFRTSAAALA AQNFTMPALS PTMTEGNIAT WRVKEGDKFS AGDVLLEIET DKATMDVEAQ DDGVMVKIM KNDGAKGVAV GARIAVIAEE GDDISSLEIP ADAAPQSKPA ESAPSAPPPP TTADQSNVAV PESAPQNASS K SAPKPPKR ...String: MASLTAACRI SARMAGRSVR GFRTSAAALA AQNFTMPALS PTMTEGNIAT WRVKEGDKFS AGDVLLEIET DKATMDVEAQ DDGVMVKIM KNDGAKGVAV GARIAVIAEE GDDISSLEIP ADAAPQSKPA ESAPSAPPPP TTADQSNVAV PESAPQNASS K SAPKPPKR QYPHYPSVAH LLKVNGIDAA AVKDITPTGP GGRLLKGDVL AYLGKINAQT PSTVSERFEK QSHLDLSNIK VA KSTEAVK ATTEKAQSKK LDAPAPPPVA VVTAPISLSA AIDVQNKLHK TIGVFLPLST FITRATEIAN QKLPLPANYQ PTA DELFNQ VLGLDKVTRK ESRGSYTPTF GSFVAPQRAA RKADIIDILA APSTRVAASA QSKSAAPGLT TSGPNVFSLQ VPKS EEKRA QAFLQKMKLV LEQEPDKLVR A UniProtKB: Pyruvate dehydrogenase X component |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 604402 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||
| Output model |  PDB-8ohs: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)