[English] 日本語
 Yorodumi
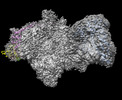
Yorodumi- EMDB-16837: Structure of the mammalian Pol II-SPT6-Elongin complex, lacking E... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the mammalian Pol II-SPT6-Elongin complex, lacking ELOA latch (composite map 2) | ||||||||||||
 Map data Map data | composite map for Pol II-SPT6-Elongin (NoN) model | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Transcription elongation / Elongin / RNA polymerase II / TRANSCRIPTION | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of isotype switching / regulation of muscle cell differentiation / Formation of RNA Pol II elongation complex / Formation of the Early Elongation Complex / Transcriptional regulation by small RNAs / RNA Polymerase II Pre-transcription Events / TP53 Regulates Transcription of DNA Repair Genes / FGFR2 alternative splicing / RNA polymerase II transcribes snRNA genes / mRNA Capping ...regulation of isotype switching / regulation of muscle cell differentiation / Formation of RNA Pol II elongation complex / Formation of the Early Elongation Complex / Transcriptional regulation by small RNAs / RNA Polymerase II Pre-transcription Events / TP53 Regulates Transcription of DNA Repair Genes / FGFR2 alternative splicing / RNA polymerase II transcribes snRNA genes / mRNA Capping / mRNA Splicing - Minor Pathway / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Elongation / RNA Polymerase II Transcription Initiation And Promoter Clearance / RNA Pol II CTD phosphorylation and interaction with CE / Estrogen-dependent gene expression / Formation of TC-NER Pre-Incision Complex / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / mRNA Splicing - Major Pathway / nucleosome organization / blastocyst formation / target-directed miRNA degradation / elongin complex / VCB complex / Cul5-RING ubiquitin ligase complex / Cul2-RING ubiquitin ligase complex / transcription elongation-coupled chromatin remodeling / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / site of DNA damage / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / mRNA transport / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / RNA polymerase I complex / transcription elongation by RNA polymerase I / RNA polymerase III complex / Formation of HIV elongation complex in the absence of HIV Tat / tRNA transcription by RNA polymerase III / RNA polymerase II, core complex / nucleosome binding / transcription by RNA polymerase I / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / transcription-coupled nucleotide-excision repair / translation initiation factor binding / RNA Polymerase II Pre-transcription Events / DNA-directed RNA polymerase complex / RNA splicing / positive regulation of RNA splicing / transcription corepressor binding / transcription elongation factor complex / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / promoter-specific chromatin binding / DNA-templated transcription initiation / Vif-mediated degradation of APOBEC3G / Inactivation of CSF3 (G-CSF) signaling / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Evasion by RSV of host interferon responses / ribonucleoside binding / Regulation of expression of SLITs and ROBOs / fibrillar center / mRNA processing / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / Antigen processing: Ubiquitination & Proteasome degradation / chromosome / Neddylation / protein-containing complex assembly / ubiquitin-dependent protein catabolic process / Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters / histone binding / protein-macromolecule adaptor activity / nucleic acid binding / transcription by RNA polymerase II / chromosome, telomeric region / protein dimerization activity / nuclear speck / protein ubiquitination / hydrolase activity / RNA-directed RNA polymerase / nucleotide binding / RNA-directed RNA polymerase activity / ubiquitin protein ligase binding / DNA-templated transcription / chromatin binding / regulation of transcription by RNA polymerase II / nucleolus / mitochondrion / extracellular space / DNA binding Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.86 Å | ||||||||||||
 Authors Authors | Chen Y / Kokic G / Dienemann C / Dybkov O / Urlaub H / Cramer P | ||||||||||||
| Funding support | European Union,  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Structure of the transcribing RNA polymerase II-Elongin complex. Authors: Ying Chen / Goran Kokic / Christian Dienemann / Olexandr Dybkov / Henning Urlaub / Patrick Cramer /   Abstract: Elongin is a heterotrimeric elongation factor for RNA polymerase (Pol) II transcription that is conserved among metazoa. Here, we report three cryo-EM structures of human Elongin bound to ...Elongin is a heterotrimeric elongation factor for RNA polymerase (Pol) II transcription that is conserved among metazoa. Here, we report three cryo-EM structures of human Elongin bound to transcribing Pol II. The structures show that Elongin subunit ELOA binds the RPB2 side of Pol II and anchors the ELOB-ELOC subunit heterodimer. ELOA contains a 'latch' that binds between the end of the Pol II bridge helix and funnel helices, thereby inducing a conformational change near the polymerase active center. The latch is required for the elongation-stimulatory activity of Elongin, but not for Pol II binding, indicating that Elongin functions by allosterically regulating the conformational mobility of the polymerase active center. Elongin binding to Pol II is incompatible with association of the super elongation complex, PAF1 complex and RTF1, which also contain an elongation-stimulatory latch element. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16837.map.gz emd_16837.map.gz | 303.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16837-v30.xml emd-16837-v30.xml emd-16837.xml emd-16837.xml | 34.8 KB 34.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16837.png emd_16837.png | 117.4 KB | ||
| Filedesc metadata |  emd-16837.cif.gz emd-16837.cif.gz | 11.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16837 http://ftp.pdbj.org/pub/emdb/structures/EMD-16837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16837 | HTTPS FTP |
-Related structure data
| Related structure data |  8oevMC  8oeuC  8oewC  8of0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16837.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16837.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | composite map for Pol II-SPT6-Elongin (NoN) model | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : The Pol II-SPT6-Elongin transcription elongation complex
+Supramolecule #1: The Pol II-SPT6-Elongin transcription elongation complex
+Macromolecule #1: DNA-directed RNA polymerase II subunit RPB1
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase II subunit RPB3
+Macromolecule #4: RNA polymerase II subunit D
+Macromolecule #5: DNA-directed RNA polymerase II subunit E
+Macromolecule #6: DNA-directed RNA polymerase II subunit F
+Macromolecule #7: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #8: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB9
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC5
+Macromolecule #11: DNA-directed RNA polymerase II subunit RPB11-a
+Macromolecule #12: RNA polymerase II subunit K
+Macromolecule #16: Elongin-A
+Macromolecule #17: Elongin-C
+Macromolecule #18: Elongin-B
+Macromolecule #19: Transcription elongation factor SPT6
+Macromolecule #13: Non-Template DNA
+Macromolecule #15: Template DNA
+Macromolecule #14: RNA
+Macromolecule #20: MAGNESIUM ION
+Macromolecule #21: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R3.5/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2.1 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.09 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 7.5 µm / Nominal defocus min: 0.35000000000000003 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.86 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 118642 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8oev: |
 Movie
Movie Controller
Controller































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)




















 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)