[English] 日本語
 Yorodumi
Yorodumi- EMDB-16817: Chaetomium thermophilum Get1/Get2 heterotetramer in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Chaetomium thermophilum Get1/Get2 heterotetramer in complex with a Get3 dimer (amphipol) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein insertion / GET pathway / tail anchored membrane protein / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationGET complex / tail-anchored membrane protein insertion into ER membrane / retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum / protein-membrane adaptor activity / endoplasmic reticulum membrane / ATP hydrolysis activity / ATP binding / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Thermochaetoides thermophila (fungus) / Thermochaetoides thermophila (fungus) /  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) | |||||||||
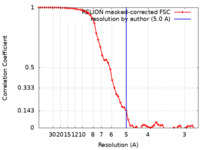
| Method | single particle reconstruction / cryo EM / Resolution: 5.0 Å | |||||||||
 Authors Authors | McDowell MA / Wild K / Sinning I | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: The GET insertase exhibits conformational plasticity and induces membrane thinning. Authors: Melanie A McDowell / Michael Heimes / Giray Enkavi / Ákos Farkas / Daniel Saar / Klemens Wild / Blanche Schwappach / Ilpo Vattulainen / Irmgard Sinning /   Abstract: The eukaryotic guided entry of tail-anchored proteins (GET) pathway mediates the biogenesis of tail-anchored (TA) membrane proteins at the endoplasmic reticulum. In the cytosol, the Get3 chaperone ...The eukaryotic guided entry of tail-anchored proteins (GET) pathway mediates the biogenesis of tail-anchored (TA) membrane proteins at the endoplasmic reticulum. In the cytosol, the Get3 chaperone captures the TA protein substrate and delivers it to the Get1/Get2 membrane protein complex (GET insertase), which then inserts the substrate via a membrane-embedded hydrophilic groove. Here, we present structures, atomistic simulations and functional data of human and Chaetomium thermophilum Get1/Get2/Get3. The core fold of the GET insertase is conserved throughout eukaryotes, whilst thinning of the lipid bilayer occurs in the vicinity of the hydrophilic groove to presumably lower the energetic barrier of membrane insertion. We show that the gating interaction between Get2 helix α3' and Get3 drives conformational changes in both Get3 and the Get1/Get2 membrane heterotetramer. Thus, we provide a framework to understand the conformational plasticity of the GET insertase and how it remodels its membrane environment to promote substrate insertion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16817.map.gz emd_16817.map.gz | 14.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16817-v30.xml emd-16817-v30.xml emd-16817.xml emd-16817.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16817_fsc.xml emd_16817_fsc.xml | 5.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_16817.png emd_16817.png | 47.9 KB | ||
| Filedesc metadata |  emd-16817.cif.gz emd-16817.cif.gz | 6.7 KB | ||
| Others |  emd_16817_half_map_1.map.gz emd_16817_half_map_1.map.gz emd_16817_half_map_2.map.gz emd_16817_half_map_2.map.gz | 13.8 MB 13.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16817 http://ftp.pdbj.org/pub/emdb/structures/EMD-16817 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16817 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16817 | HTTPS FTP |
-Validation report
| Summary document |  emd_16817_validation.pdf.gz emd_16817_validation.pdf.gz | 717.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16817_full_validation.pdf.gz emd_16817_full_validation.pdf.gz | 716.9 KB | Display | |
| Data in XML |  emd_16817_validation.xml.gz emd_16817_validation.xml.gz | 10.7 KB | Display | |
| Data in CIF |  emd_16817_validation.cif.gz emd_16817_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16817 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16817 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16817 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16817 | HTTPS FTP |
-Related structure data
| Related structure data |  8oduMC  8cqzC  8cr1C  8cr2C  8odvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16817.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16817.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.375 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_16817_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16817_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Chaetomium thermophilum Get1/Get2 heterotetramer in complex with ...
| Entire | Name: Chaetomium thermophilum Get1/Get2 heterotetramer in complex with a Get3 dimer |
|---|---|
| Components |
|
-Supramolecule #1: Chaetomium thermophilum Get1/Get2 heterotetramer in complex with ...
| Supramolecule | Name: Chaetomium thermophilum Get1/Get2 heterotetramer in complex with a Get3 dimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Get2-Get1 was expressed as a fusion protein in S. cerevisiae and Get3 was expressed in E. coli. The complex components were purified and reconstituted in vitro. |
|---|---|
| Molecular weight | Theoretical: 165 KDa |
-Supramolecule #2: Tail-anchored protein insertase Get1 and Get2
| Supramolecule | Name: Tail-anchored protein insertase Get1 and Get2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) |
-Supramolecule #3: Dimeric ATPase Get3
| Supramolecule | Name: Dimeric ATPase Get3 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) |
-Macromolecule #1: ATPase GET3
| Macromolecule | Name: ATPase GET3 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: Hydrolases; Acting on acid anhydrides |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) |
| Molecular weight | Theoretical: 37.127391 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEPTLQSILD QRSLRWIFVG GKGGVGKTTT SCSLAIQLAK VRRSVLLLST DPAHNLSDAF SQKFGKEARL VEGFDNLYAM EIDPNGSMQ DLLAGQTGDG DAGMGGVGVM QDLAYAIPGI DEAMSFAEVL KQVNSLSYET IVFDTAPTGH TLRFLQFPTV L EKALAKVS ...String: MEPTLQSILD QRSLRWIFVG GKGGVGKTTT SCSLAIQLAK VRRSVLLLST DPAHNLSDAF SQKFGKEARL VEGFDNLYAM EIDPNGSMQ DLLAGQTGDG DAGMGGVGVM QDLAYAIPGI DEAMSFAEVL KQVNSLSYET IVFDTAPTGH TLRFLQFPTV L EKALAKVS QLSGQYGSLL NGILGGSGTL PNGQTLSDVM EKLDSLRVTI SEVNAQFKDE RLTTFVCVCI PEFLSLYETE RM IQELANY GIDTHCIVVN QLLFPKPGSD CEQCTARRRM QKKYLDQIEE LYDEEFNVVK MPLLVEEVRG KERLEKFSEM LIK PFVPPE WSHPQFEK UniProtKB: Arsenite translocating ATPase-like protein |
-Macromolecule #2: Protein GET2,Protein GET1
| Macromolecule | Name: Protein GET2,Protein GET1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) |
| Molecular weight | Theoretical: 45.573727 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGRPTPLWRF LHTLLAVALG LAVIMLSPFG GTKLERDRAA AAVAGSASER EWLASLTDSY PLVKTGLGGG LFWAFATGEA ILLGTRWLF LSKKKKAATA AAKVNNNNGE GDDAELDSVE QAIELALEFF PAIRQPVEYL RPKVAVAMRY VDVGMTLWRD V MLALFVLG ...String: MGRPTPLWRF LHTLLAVALG LAVIMLSPFG GTKLERDRAA AAVAGSASER EWLASLTDSY PLVKTGLGGG LFWAFATGEA ILLGTRWLF LSKKKKAATA AAKVNNNNGE GDDAELDSVE QAIELALEFF PAIRQPVEYL RPKVAVAMRY VDVGMTLWRD V MLALFVLG AVAWWRAGSG SENLYFQSGS GSMSLLLVIF LLELVVQLVN TIGAKTINNL LWRFYLSIPG SPLAKDFAEQ RA KQKEYLQ VRHDLNATSS QDEFAKWARL QRKHDKLMDE LEKKKSQLDA HRTSFSRKLT IYRWILTRGM QWFLCFWFSS QPM FWLPYG WFPYWVEWLV SFPNAPMGSV SIVVWQSACS GVLALVIEAV MAVVRYTGGT GMQKQRQPVP AAGGAPGTSK KDLG SGSLE VLFQ UniProtKB: Uncharacterized protein, Protein GET1 |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.4 mg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE | ||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 279 K / Instrument: FEI VITROBOT MARK IV | ||||||
| Details | Complex stabilised in A835 amphipol |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 5599 / Average exposure time: 4.0 sec. / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||
| Output model |  PDB-8odu: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)