+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | TniQ-capped Tns-ATP-dsDNA complex | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Transposition / TniQ / TnsC / CRISPR-Cas / Tn7-like transposon / DNA BINDING PROTEIN | |||||||||||||||
| Function / homology | Bacterial TniB / Bacterial TniB protein / : / TniQ / TniQ / P-loop containing nucleoside triphosphate hydrolase / TnsC / TniQ (Homology model) Function and homology information Function and homology information | |||||||||||||||
| Biological species |  Scytonema hofmannii (bacteria) / synthetic construct (others) Scytonema hofmannii (bacteria) / synthetic construct (others) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.44 Å | |||||||||||||||
 Authors Authors | Querques I / Schmitz M / Oberli S / Chanez C / Jinek M | |||||||||||||||
| Funding support | European Union,  Switzerland, Switzerland,  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Structural basis for the assembly of the type V CRISPR-associated transposon complex. Authors: Michael Schmitz / Irma Querques / Seraina Oberli / Christelle Chanez / Martin Jinek /  Abstract: CRISPR-Cas systems have been co-opted by Tn7-like transposable elements to direct RNA-guided transposition. Type V-K CRISPR-associated transposons rely on the concerted activities of the ...CRISPR-Cas systems have been co-opted by Tn7-like transposable elements to direct RNA-guided transposition. Type V-K CRISPR-associated transposons rely on the concerted activities of the pseudonuclease Cas12k, the AAA+ ATPase TnsC, the Zn-finger protein TniQ, and the transposase TnsB. Here we present a cryo-electron microscopic structure of a target DNA-bound Cas12k-transposon recruitment complex comprised of RNA-guided Cas12k, TniQ, a polymeric TnsC filament and, unexpectedly, the ribosomal protein S15. Complex assembly, mediated by a network of interactions involving the guide RNA, TniQ, and S15, results in R-loop completion. TniQ contacts two TnsC protomers at the Cas12k-proximal filament end, likely nucleating its polymerization. Transposition activity assays corroborate our structural findings, implying that S15 is a bona fide component of the type V crRNA-guided transposon machinery. Altogether, our work uncovers key mechanistic aspects underpinning RNA-mediated assembly of CRISPR-associated transposons to guide their development as programmable tools for site-specific insertion of large DNA payloads. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15974.map.gz emd_15974.map.gz | 778.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15974-v30.xml emd-15974-v30.xml emd-15974.xml emd-15974.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_15974.png emd_15974.png | 110.1 KB | ||
| Filedesc metadata |  emd-15974.cif.gz emd-15974.cif.gz | 6 KB | ||
| Others |  emd_15974_half_map_1.map.gz emd_15974_half_map_1.map.gz emd_15974_half_map_2.map.gz emd_15974_half_map_2.map.gz | 763.6 MB 763.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15974 http://ftp.pdbj.org/pub/emdb/structures/EMD-15974 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15974 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15974 | HTTPS FTP |
-Validation report
| Summary document |  emd_15974_validation.pdf.gz emd_15974_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15974_full_validation.pdf.gz emd_15974_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_15974_validation.xml.gz emd_15974_validation.xml.gz | 20.3 KB | Display | |
| Data in CIF |  emd_15974_validation.cif.gz emd_15974_validation.cif.gz | 23.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15974 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15974 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15974 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15974 | HTTPS FTP |
-Related structure data
| Related structure data |  8bd4MC  8bd5C  8bd6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15974.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15974.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.65 Å | ||||||||||||||||||||||||||||||||||||
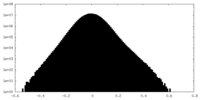
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_15974_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
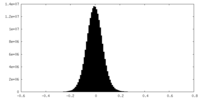
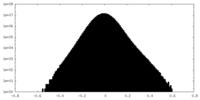
| Density Histograms |
-Half map: #1
| File | emd_15974_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
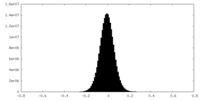
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of TnsC and TniQ transposon proteins bound to ATP and dsDNA
| Entire | Name: Complex of TnsC and TniQ transposon proteins bound to ATP and dsDNA |
|---|---|
| Components |
|
-Supramolecule #1: Complex of TnsC and TniQ transposon proteins bound to ATP and dsDNA
| Supramolecule | Name: Complex of TnsC and TniQ transposon proteins bound to ATP and dsDNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 / Details: TniQ, TnsC, dsDNA |
|---|---|
| Source (natural) | Organism:  Scytonema hofmannii (bacteria) Scytonema hofmannii (bacteria) |
-Macromolecule #1: TnsC
| Macromolecule | Name: TnsC / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Scytonema hofmannii (bacteria) Scytonema hofmannii (bacteria) |
| Molecular weight | Theoretical: 31.444617 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTEAQAIAKQ LGGVKPDDEW LQAEIARLKG KSIVPLQQVK TLHDWLDGKR KARKSCRVVG ESRTGKTVAC DAYRYRHKPQ QEAGRPPTV PVVYIRPHQK CGPKDLFKKI TEYLKYRVTK GTVSDFRDRT IEVLKGCGVE MLIIDEADRL KPETFADVRD I AEDLGIAV ...String: MTEAQAIAKQ LGGVKPDDEW LQAEIARLKG KSIVPLQQVK TLHDWLDGKR KARKSCRVVG ESRTGKTVAC DAYRYRHKPQ QEAGRPPTV PVVYIRPHQK CGPKDLFKKI TEYLKYRVTK GTVSDFRDRT IEVLKGCGVE MLIIDEADRL KPETFADVRD I AEDLGIAV VLVGTDRLDA VIKRDEQVLE RFRAHLRFGK LSGEDFKNTV EMWEQMVLKL PVSSNLKSKE MLRILTSATE GY IGRLDEI LREAAIRSLS RGLKKIDKAV LQEVAKEYK UniProtKB: TnsC |
-Macromolecule #2: TniQ (Homology model)
| Macromolecule | Name: TniQ (Homology model) / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Scytonema hofmannii (bacteria) Scytonema hofmannii (bacteria) |
| Molecular weight | Theoretical: 19.01124 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIEAPDVKPW LFLIKPYEGE SLSHFLGRFR RANHLSASGL GTLAGIGAIV ARWERFHFNP RPSQQELEAI ASVVEVDAQR LAQMLPPAG VGMQHEPIRL CGACYAESPC HRIEWQYKSV WKCDRHQLKI LAKCPNCQAP FKMPALWEDG CCHRCRMPFA E MAKLQKV UniProtKB: TniQ (Homology model) |
-Macromolecule #3: DNA (5'-D(P*GP*AP*TP*CP*GP*AP*TP*CP*GP*AP*TP*CP*GP*AP*TP*C)-3')
| Macromolecule | Name: DNA (5'-D(P*GP*AP*TP*CP*GP*AP*TP*CP*GP*AP*TP*CP*GP*AP*TP*C)-3') type: dna / ID: 3 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.898191 KDa |
| Sequence | String: (DG)(DA)(DT)(DC)(DG)(DA)(DT)(DC)(DG)(DA) (DT)(DC)(DG)(DA)(DT)(DC) |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 7 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 7 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 6 / Number of copies: 6 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 66.036 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.44 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 35964 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)