登録情報 データベース : EMDB / ID : EMD-15770タイトル Type I amyloid-beta 42 filaments from high-spin supernatants of aqueous extracts from Alzheimer's disease brains | ABeta42 組織 : Type I amyloid-beta 42 filaments in soluble high-molecular weight aggregate fractions extracted from Alzheimer's disease brainタンパク質・ペプチド : Amyloid-beta precursor protein / / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
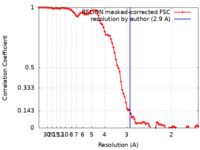
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / / 解像度 : 2.9 Å Yang Y / Stern MA / Meunier LA / Liu W / Cai YQ / Ericsson M / Liu L / Selkoe JD / Goedert M / Scheres HWS 資金援助 Organization Grant number 国 Medical Research Council (MRC, United Kingdom) MC_UP_1201/25 Medical Research Council (MRC, United Kingdom) MC_UP_A025_1013 Medical Research Council (MRC, United Kingdom) MC_U105184291
ジャーナル : Neuron / 年 : 2023タイトル : Abundant Aβ fibrils in ultracentrifugal supernatants of aqueous extracts from Alzheimer's disease brains.著者 : Andrew M Stern / Yang Yang / Shanxue Jin / Keitaro Yamashita / Angela L Meunier / Wen Liu / Yuqi Cai / Maria Ericsson / Lei Liu / Michel Goedert / Sjors H W Scheres / Dennis J Selkoe / 要旨 : Soluble oligomers of amyloid β-protein (Aβ) have been defined as aggregates in supernatants following ultracentrifugation of aqueous extracts from Alzheimer's disease (AD) brains and are believed ... Soluble oligomers of amyloid β-protein (Aβ) have been defined as aggregates in supernatants following ultracentrifugation of aqueous extracts from Alzheimer's disease (AD) brains and are believed to be upstream initiators of synaptic dysfunction, but little is known about their structures. We now report the unexpected presence of Aβ fibrils in synaptotoxic high-speed supernatants from AD brains extracted by soaking in an aqueous buffer. The fibrils did not appear to form during preparation, and their counts by EM correlated with Aβ ELISA quantification. Cryo-EM structures of aqueous Aβ fibrils were identical to those from sarkosyl-insoluble homogenates. The fibrils in aqueous extracts were labeled by lecanemab, an Aβ aggregate-directed antibody reported to improve AD cognitive outcomes. Lecanemab provided protection against aqueous fibril synaptotoxicity. We conclude that fibrils are abundant in aqueous extracts from AD brains and have the same structures as those from plaques. These findings have implications for AD pathogenesis and drug design. 履歴 登録 2022年9月6日 - ヘッダ(付随情報) 公開 2022年11月2日 - マップ公開 2022年11月2日 - 更新 2024年7月24日 - 現状 2024年7月24日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報
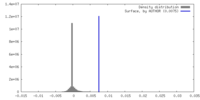
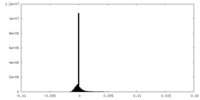
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 英国, 3件
英国, 3件  引用
引用 ジャーナル: Neuron / 年: 2023
ジャーナル: Neuron / 年: 2023

 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_15770.map.gz
emd_15770.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-15770-v30.xml
emd-15770-v30.xml emd-15770.xml
emd-15770.xml EMDBヘッダ
EMDBヘッダ emd_15770_fsc.xml
emd_15770_fsc.xml FSCデータファイル
FSCデータファイル emd_15770.png
emd_15770.png emd_15770_msk_1.map
emd_15770_msk_1.map マスクマップ
マスクマップ emd-15770.cif.gz
emd-15770.cif.gz emd_15770_half_map_1.map.gz
emd_15770_half_map_1.map.gz emd_15770_half_map_2.map.gz
emd_15770_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-15770
http://ftp.pdbj.org/pub/emdb/structures/EMD-15770 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15770
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15770 emd_15770_validation.pdf.gz
emd_15770_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_15770_full_validation.pdf.gz
emd_15770_full_validation.pdf.gz emd_15770_validation.xml.gz
emd_15770_validation.xml.gz emd_15770_validation.cif.gz
emd_15770_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15770
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15770 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15770
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15770 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_15770.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_15770.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) emd_15770_msk_1.map
emd_15770_msk_1.map 試料の構成要素
試料の構成要素 Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 ムービー
ムービー コントローラー
コントローラー
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)