+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Vaccinia C16 protein bound to Ku70/Ku80 | |||||||||||||||||||||
 Map data Map data | Map of C16 C-terminal domains bound to Ku70/Ku80 | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | C16 vaccinia virus protein Ku70/Ku80 DNA binding inhibition / VIRAL PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationKu70:Ku80 complex / negative regulation of t-circle formation / DNA end binding / small-subunit processome assembly / positive regulation of lymphocyte differentiation / DNA-dependent protein kinase complex / DNA-dependent protein kinase-DNA ligase 4 complex / nonhomologous end joining complex / regulation of smooth muscle cell proliferation / cellular response to X-ray ...Ku70:Ku80 complex / negative regulation of t-circle formation / DNA end binding / small-subunit processome assembly / positive regulation of lymphocyte differentiation / DNA-dependent protein kinase complex / DNA-dependent protein kinase-DNA ligase 4 complex / nonhomologous end joining complex / regulation of smooth muscle cell proliferation / cellular response to X-ray / double-strand break repair via classical nonhomologous end joining / nuclear telomere cap complex / Cytosolic sensors of pathogen-associated DNA / IRF3-mediated induction of type I IFN / recombinational repair / positive regulation of neurogenesis / regulation of telomere maintenance / U3 snoRNA binding / protein localization to chromosome, telomeric region / cellular hyperosmotic salinity response / 2-LTR circle formation / hematopoietic stem cell proliferation / telomeric DNA binding / positive regulation of protein kinase activity / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / site of DNA damage / 5'-deoxyribose-5-phosphate lyase activity / hematopoietic stem cell differentiation / ATP-dependent activity, acting on DNA / telomere maintenance via telomerase / neurogenesis / activation of innate immune response / DNA helicase activity / telomere maintenance / cyclin binding / cellular response to leukemia inhibitory factor / Nonhomologous End-Joining (NHEJ) / small-subunit processome / enzyme activator activity / protein-DNA complex / cellular response to gamma radiation / double-strand break repair via nonhomologous end joining / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / double-strand break repair / double-stranded DNA binding / scaffold protein binding / secretory granule lumen / DNA recombination / transcription regulator complex / ficolin-1-rich granule lumen / damaged DNA binding / chromosome, telomeric region / transcription cis-regulatory region binding / ribonucleoprotein complex / innate immune response / negative regulation of DNA-templated transcription / DNA damage response / ubiquitin protein ligase binding / Neutrophil degranulation / positive regulation of DNA-templated transcription / protein-containing complex binding / nucleolus / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / protein-containing complex / DNA binding / RNA binding / extracellular region / nucleoplasm / ATP binding / nucleus / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Vaccinia virus Western Reserve Vaccinia virus Western Reserve | |||||||||||||||||||||
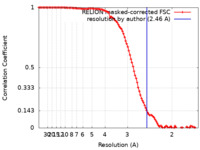
| Method | single particle reconstruction / cryo EM / Resolution: 2.46 Å | |||||||||||||||||||||
 Authors Authors | Rivera-Calzada A / Arribas-Bosacoma R / Pearl LH / Llorca O | |||||||||||||||||||||
| Funding support |  Spain, Spain,  United Kingdom, 6 items United Kingdom, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural basis for the inactivation of cytosolic DNA sensing by the vaccinia virus. Authors: Angel Rivera-Calzada / Raquel Arribas-Bosacoma / Alba Ruiz-Ramos / Paloma Escudero-Bravo / Jasminka Boskovic / Rafael Fernandez-Leiro / Antony W Oliver / Laurence H Pearl / Oscar Llorca /   Abstract: Detection of cytosolic DNA is a central element of the innate immunity system against viral infection. The Ku heterodimer, a component of the NHEJ pathway of DNA repair in the nucleus, functions as ...Detection of cytosolic DNA is a central element of the innate immunity system against viral infection. The Ku heterodimer, a component of the NHEJ pathway of DNA repair in the nucleus, functions as DNA sensor that detects dsDNA of viruses that replicate in the cytoplasm. Vaccinia virus expresses two proteins, C4 and C16, that inactivate DNA sensing and enhance virulence. The structural basis for this is unknown. Here we determine the structure of the C16 - Ku complex using cryoEM. Ku binds dsDNA by a preformed ring but C16 sterically blocks this access route, abrogating binding to a dsDNA end and its insertion into DNA-PK, thereby averting signalling into the downstream innate immunity system. C4 replicates these activities using a domain with 54% identity to C16. Our results reveal how vaccinia virus subverts the capacity of Ku to recognize viral DNA. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15415.map.gz emd_15415.map.gz | 90.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15415-v30.xml emd-15415-v30.xml emd-15415.xml emd-15415.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15415_fsc.xml emd_15415_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_15415.png emd_15415.png | 87.3 KB | ||
| Filedesc metadata |  emd-15415.cif.gz emd-15415.cif.gz | 7.6 KB | ||
| Others |  emd_15415_half_map_1.map.gz emd_15415_half_map_1.map.gz emd_15415_half_map_2.map.gz emd_15415_half_map_2.map.gz | 84.1 MB 84.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15415 http://ftp.pdbj.org/pub/emdb/structures/EMD-15415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15415 | HTTPS FTP |
-Related structure data
| Related structure data |  8ag4MC  8ag3C  8ag5C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15415.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15415.map.gz / Format: CCP4 / Size: 107.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of C16 C-terminal domains bound to Ku70/Ku80 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.835 Å | ||||||||||||||||||||||||||||||||||||
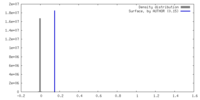
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map 1
| File | emd_15415_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
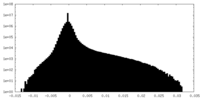
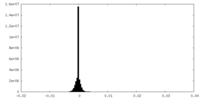
| Density Histograms |
-Half map: Half map 2
| File | emd_15415_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
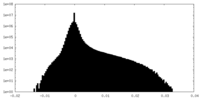
| Density Histograms |
- Sample components
Sample components
-Entire : Vaccinia C16 protein bound to Ku70/Ku80
| Entire | Name: Vaccinia C16 protein bound to Ku70/Ku80 |
|---|---|
| Components |
|
-Supramolecule #1: Vaccinia C16 protein bound to Ku70/Ku80
| Supramolecule | Name: Vaccinia C16 protein bound to Ku70/Ku80 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 230 KDa |
-Macromolecule #1: X-ray repair cross-complementing protein 6
| Macromolecule | Name: X-ray repair cross-complementing protein 6 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.234703 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSAWSHPQFE KGSAGSAAGS GAGWSHPQFE KLEVLFQGPG GSMSGWESYY KTEGDEEAEE EQEENLEASG DYKYSGRDSL IFLVDASKA MFESQSEDEL TPFDMSIQCI QSVYISKIIS SDRDLLAVVF YGTEKDKNSV NFKNIYVLQE LDNPGAKRIL E LDQFKGQQ ...String: MSAWSHPQFE KGSAGSAAGS GAGWSHPQFE KLEVLFQGPG GSMSGWESYY KTEGDEEAEE EQEENLEASG DYKYSGRDSL IFLVDASKA MFESQSEDEL TPFDMSIQCI QSVYISKIIS SDRDLLAVVF YGTEKDKNSV NFKNIYVLQE LDNPGAKRIL E LDQFKGQQ GQKRFQDMMG HGSDYSLSEV LWVCANLFSD VQFKMSHKRI MLFTNEDNPH GNDSAKASRA RTKAGDLRDT GI FLDLMHL KKPGGFDISL FYRDIISIAE DEDLRVHFEE SSKLEDLLRK VRAKETRKRA LSRLKLKLNK DIVISVGIYN LVQ KALKPP PIKLYRETNE PVKTKTRTFN TSTGGLLLPS DTKRSQIYGS RQIILEKEET EELKRFDDPG LMLMGFKPLV LLKK HHYLR PSLFVYPEES LVIGSSTLFS ALLIKCLEKE VAALCRYTPR RNIPPYFVAL VPQEEELDDQ KIQVTPPGFQ LVFLP FADD KRKMPFTEKI MATPEQVGKM KAIVEKLRFT YRSDSFENPV LQQHFRNLEA LALDLMEPEQ AVDLTLPKVE AMNKRL GSL VDEFKELVYP PDYNPEGKVT KRKHDNEGSG SKRPKVEYSE EELKTHISKG TLGKFTVPML KEACRAYGLK SGLKKQE LL EALTKHFQD UniProtKB: X-ray repair cross-complementing protein 6 |
-Macromolecule #2: X-ray repair cross-complementing protein 5
| Macromolecule | Name: X-ray repair cross-complementing protein 5 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 85.546484 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MAHHHHHHHH HHGALEVLFQ GPHMVRSGNK AAVVLCMDVG FTMSNSIPGI ESPFEQAKKV ITMFVQRQVF AENKDEIALV LFGTDGTDN PLSGGDQYQN ITVHRHLMLP DFDLLEDIES KIQPGSQQAD FLDALIVSMD VIQHETIGKK FEKRHIEIFT D LSSRFSKS ...String: MAHHHHHHHH HHGALEVLFQ GPHMVRSGNK AAVVLCMDVG FTMSNSIPGI ESPFEQAKKV ITMFVQRQVF AENKDEIALV LFGTDGTDN PLSGGDQYQN ITVHRHLMLP DFDLLEDIES KIQPGSQQAD FLDALIVSMD VIQHETIGKK FEKRHIEIFT D LSSRFSKS QLDIIIHSLK KCDISLQFFL PFSLGKEDGS GDRGDGPFRL GGHGPSFPLK GITEQQKEGL EIVKMVMISL EG EDGLDEI YSFSESLRKL CVFKKIERHS IHWPCRLTIG SNLSIRIAAY KSILQERVKK TWTVVDAKTL KKEDIQKETV YCL NDDDET EVLKEDIIQG FRYGSDIVPF SKVDEEQMKY KSEGKCFSVL GFCKSSQVQR RFFMGNQVLK VFAARDDEAA AVAL SSLIH ALDDLDMVAI VRYAYDKRAN PQVGVAFPHI KHNYECLVYV QLPFMEDLRQ YMFSSLKNSK KYAPTEAQLN AVDAL IDSM SLAKKDEKTD TLEDLFPTTK IPNPRFQRLF QCLLHRALHP REPLPPIQQH IWNMLNPPAE VTTKSQIPLS KIKTLF PLI EAKKKDQVTA QEIFQDNHED GPTAKKLKTE QGGAHFSVSS LAEGSVTSVG SVNPAENFRV LVKQKKASFE EASNQLI NH IEQFLDTNET PYFMKSIDCI RAFREEAIKF SEEQRFNNFL KALQEKVEIK QLNHFWEIVV QDGITLITKE EASGSSVT A EEAKKFLAPK DKPSGDTAAV FEEGGDVDDL LDMI UniProtKB: X-ray repair cross-complementing protein 5 |
-Macromolecule #3: Protein C10
| Macromolecule | Name: Protein C10 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Vaccinia virus Western Reserve / Strain: Western Reserve Vaccinia virus Western Reserve / Strain: Western Reserve |
| Molecular weight | Theoretical: 42.47668 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDIYDDKGLQ TIKLFNNEFD CIRNDIRELF KHVTDSDSIQ LPMEDNSDII ENIRKILYRR LKNVECVDID STITFMKYDP NDDNKRTCS NWVPLTNNYM EYCLVIYLET PICGGKIKLY HPTGNIKSDK DIMFAKTLDF KSKKVLTGRK TIAVLDISVS Y NRSMTTIH ...String: MDIYDDKGLQ TIKLFNNEFD CIRNDIRELF KHVTDSDSIQ LPMEDNSDII ENIRKILYRR LKNVECVDID STITFMKYDP NDDNKRTCS NWVPLTNNYM EYCLVIYLET PICGGKIKLY HPTGNIKSDK DIMFAKTLDF KSKKVLTGRK TIAVLDISVS Y NRSMTTIH YNDDVDIDIH TDKNGKELCY CYITIDDHYL VDVETIGVIV NRSGKCLLVN NHLGIGIVKD KRISDSFGDV CM DTIFDFS EARELFSLTN DDNRNIAWDT DKLDDDTDIW TPVTEDDYKF LSRLVLYAKS QSDTVFDYYV LTGDTEPPTV FIF KVTRFY FNMPKGGENL YFQGWSHPQF EKGGGSGGGS GGSSAWSHPQ FEK UniProtKB: Protein C10 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.9 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 13216 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | Initial model for chains C and D was generated using AlphaFold2 | ||||||
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||
| Output model |  PDB-8ag4: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)