+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full AAV3B-VP1KO virion | |||||||||
 Map data Map data | Sharpened cryo-EM map of the full AAV3B-VP1KO particle | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Adeno-Associated Virus / AAV / AAV3B / AAV serotype 3B / Full / VP1KO / VIRUS | |||||||||
| Function / homology | Phospholipase A2-like domain / Phospholipase A2-like domain / Parvovirus coat protein VP2 / Parvovirus coat protein VP1/VP2 / Parvovirus coat protein VP1/VP2 / Capsid/spike protein, ssDNA virus / T=1 icosahedral viral capsid / structural molecule activity / Capsid protein VP1 Function and homology information Function and homology information | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Adeno-associated virus - 3 Adeno-associated virus - 3 | |||||||||
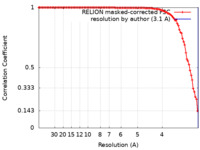
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Arriaga I / Abrescia NGA | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Hum Gene Ther / Year: 2022 Journal: Hum Gene Ther / Year: 2022Title: Cellular and Structural Characterization of VP1 and VP2 Knockout Mutants of AAV3B Serotype and Implications for AAV Manufacturing. Authors: Iker Arriaga / Aitor Navarro / Amaia Etxabe / César Trigueros / R Jude Samulski / Philippe Moullier / Achille François / Nicola G A Abrescia /   Abstract: AAV virion biology is still lacking a complete understanding of the role that the various structural subunits (VP1, 2, and 3) play in virus assembly, infectivity, and therapeutic delivery for ...AAV virion biology is still lacking a complete understanding of the role that the various structural subunits (VP1, 2, and 3) play in virus assembly, infectivity, and therapeutic delivery for clinical indications. In this study, we focus on the less studied adeno-associated virus AAV3B and generate a collection of AAV plasmid substrates that assemble virion particles deficient specifically in VP1, VP2, or VP1 and 2 structural subunits. Using a collection of biological and structural assays, we observed that virions devoid of VP1, VP2, or VP1 and 2 efficiently assembled virion particles, indistinguishable by cryoelectron microscopy (cryo-EM) from that of wild type (WT), but unique in virion transduction (WT > VP2 > VP1 > VP1 and 2 mutants). We also observed that the missing structural subunit was mostly compensated by additional VP3 protomers in the formed virion particle. Using cryo-EM analysis, virions fell into three classes, namely full, empty, and partially filled, based on comparison of density values within the capsid. Further, we characterize virions described as "broken" or "disassembled" particles, and provide structural information that supports the particle dissolution occurring through the two-fold symmetry sites. Finally, we highlight the unique value of employing cryo-EM as an essential tool for release criteria with respect to AAV manufacturing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15286.map.gz emd_15286.map.gz | 96.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15286-v30.xml emd-15286-v30.xml emd-15286.xml emd-15286.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15286_fsc.xml emd_15286_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_15286.png emd_15286.png | 196 KB | ||
| Filedesc metadata |  emd-15286.cif.gz emd-15286.cif.gz | 6.3 KB | ||
| Others |  emd_15286_additional_1.map.gz emd_15286_additional_1.map.gz emd_15286_half_map_1.map.gz emd_15286_half_map_1.map.gz emd_15286_half_map_2.map.gz emd_15286_half_map_2.map.gz | 79.1 MB 79.1 MB 79.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15286 http://ftp.pdbj.org/pub/emdb/structures/EMD-15286 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15286 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15286 | HTTPS FTP |
-Related structure data
| Related structure data |  8a9uMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15286.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15286.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened cryo-EM map of the full AAV3B-VP1KO particle | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.556 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Reconstruction of the final iteration of the 3D...
| File | emd_15286_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the final iteration of the 3D auto refinement of the full AAV3B-VP1KO particle | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half-1 map of the 3D auto refinement...
| File | emd_15286_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half-1 map of the 3D auto refinement of the full AAV3B-VP1KO particle | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half-2 map of the 3D auto refinement...
| File | emd_15286_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half-2 map of the 3D auto refinement of the full AAV3B-VP1KO particle | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Adeno-associated virus - 3
| Entire | Name:  Adeno-associated virus - 3 Adeno-associated virus - 3 |
|---|---|
| Components |
|
-Supramolecule #1: Adeno-associated virus - 3
| Supramolecule | Name: Adeno-associated virus - 3 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: For the recombinant production of the VP1KO virion, we used the Pro10TM cell line. NCBI-ID: 46350 / Sci species name: Adeno-associated virus - 3 / Sci species strain: 3B / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: Yes |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Virus shell | Shell ID: 1 / Diameter: 260.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 Details: For pXR3b-VP1KO, the ATG start codon of VP1 was changed to TGA, and the GAT codon of Asp4 was changed to GAC to remove an alternative ATG. Thus this sample only has VP2 and VP3. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.728281 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: TAPGKKRPVD QSPQEPDSSS GVGKSGKQPA RKRLNFGQTG DSESVPDPQP LGEPPAAPTS LGSNTMASGG GAPMADNNEG ADGVGNSSG NWHCDSQWLG DRVITTSTRT WALPTYNNHL YKQISSQSGA SNDNHYFGYS TPWGYFDFNR FHCHFSPRDW Q RLINNNWG ...String: TAPGKKRPVD QSPQEPDSSS GVGKSGKQPA RKRLNFGQTG DSESVPDPQP LGEPPAAPTS LGSNTMASGG GAPMADNNEG ADGVGNSSG NWHCDSQWLG DRVITTSTRT WALPTYNNHL YKQISSQSGA SNDNHYFGYS TPWGYFDFNR FHCHFSPRDW Q RLINNNWG FRPKKLSFKL FNIQVKEVTQ NDGTTTIANN LTSTVQVFTD SEYQLPYVLG SAHQGCLPPF PADVFMVPQY GY LTLNNGS QAVGRSSFYC LEYFPSQMLR TGNNFQFSYT FEDVPFHSSY AHSQSLDRLM NPLIDQYLYY LNRTQGTTSG TTN QSRLLF SQAGPQSMSL QARNWLPGPC YRQQRLSKTA NDNNNSNFPW TAASKYHLNG RDSLVNPGPA MASHKDDEEK FFPM HGNLI FGKEGTTASN AELDNVMITD EEEIRTTNPV ATEQYGTVAN NLQSSNTAPT TRTVNDQGAL PGMVWQDRDV YLQGP IWAK IPHTDGHFHP SPLMGGFGLK HPPPQIMIKN TPVPANPPTT FSPAKFASFI TQYSTGQVSV EIEWELQKEN SKRWNP EIQ YTSNYNKSVN VDFTVDTNGV YSEPRPIGTR YLTRNL UniProtKB: Capsid protein VP1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK III Details: Parameters: 2 seconds of blot time, -2 offset value, after 45 seconds of incubation. Quantifoil Cu 300-mesh R1.2/1.3 holey-carbon grids with an extra layer of carbon on top added in-house.. |
| Details | VP1KO 4.23E+13 vg/ml |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Number real images: 2384 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 92000 |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)