+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | murine perforin-2 pore on liposome by subtomogram averaging | |||||||||
 Map data Map data | main map, low pass filtered to 18 Angstrom | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 18.0 Å | |||||||||
 Authors Authors | Yu X / Ni T / Zhang P / Gilbert R | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2022 Journal: EMBO J / Year: 2022Title: Cryo-EM structures of perforin-2 in isolation and assembled on a membrane suggest a mechanism for pore formation. Authors: Xiulian Yu / Tao Ni / George Munson / Peijun Zhang / Robert J C Gilbert /   Abstract: Perforin-2 (PFN2, MPEG1) is a key pore-forming protein in mammalian innate immunity restricting intracellular bacteria proliferation. It forms a membrane-bound pre-pore complex that converts to a ...Perforin-2 (PFN2, MPEG1) is a key pore-forming protein in mammalian innate immunity restricting intracellular bacteria proliferation. It forms a membrane-bound pre-pore complex that converts to a pore-forming structure upon acidification; but its mechanism of conformational transition has been debated. Here we used cryo-electron microscopy, tomography and subtomogram averaging to determine structures of PFN2 in pre-pore and pore conformations in isolation and bound to liposomes. In isolation and upon acidification, the pre-assembled complete pre-pore rings convert to pores in both flat ring and twisted conformations. On membranes, in situ assembled PFN2 pre-pores display various degrees of completeness; whereas PFN2 pores are mainly incomplete arc structures that follow the same subunit packing arrangements as found in isolation. Both assemblies on membranes use their P2 β-hairpin for binding to the lipid membrane surface. Overall, these structural snapshots suggest a molecular mechanism for PFN2 pre-pore to pore transition on a targeted membrane, potentially using the twisted pore as an intermediate or alternative state to the flat conformation, with the capacity to cause bilayer distortion during membrane insertion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15087.map.gz emd_15087.map.gz | 88 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15087-v30.xml emd-15087-v30.xml emd-15087.xml emd-15087.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_15087.png emd_15087.png | 59 KB | ||
| Masks |  emd_15087_msk_1.map emd_15087_msk_1.map | 95 MB |  Mask map Mask map | |
| Others |  emd_15087_half_map_1.map.gz emd_15087_half_map_1.map.gz emd_15087_half_map_2.map.gz emd_15087_half_map_2.map.gz | 77.9 MB 77.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15087 http://ftp.pdbj.org/pub/emdb/structures/EMD-15087 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15087 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15087 | HTTPS FTP |
-Validation report
| Summary document |  emd_15087_validation.pdf.gz emd_15087_validation.pdf.gz | 944.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15087_full_validation.pdf.gz emd_15087_full_validation.pdf.gz | 943.9 KB | Display | |
| Data in XML |  emd_15087_validation.xml.gz emd_15087_validation.xml.gz | 13.3 KB | Display | |
| Data in CIF |  emd_15087_validation.cif.gz emd_15087_validation.cif.gz | 15.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15087 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15087 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15087 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15087 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15087.map.gz / Format: CCP4 / Size: 95 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15087.map.gz / Format: CCP4 / Size: 95 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map, low pass filtered to 18 Angstrom | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.63 Å | ||||||||||||||||||||||||||||||||||||
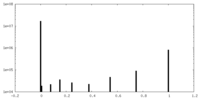
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15087_msk_1.map emd_15087_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
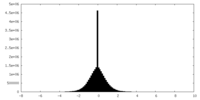
| Density Histograms |
-Half map: #1
| File | emd_15087_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
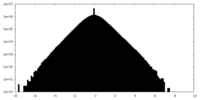
| Density Histograms |
-Half map: #2
| File | emd_15087_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
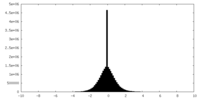
| Density Histograms |
- Sample components
Sample components
-Entire : murine perforin-2 (Mpeg1) ectodomain pore
| Entire | Name: murine perforin-2 (Mpeg1) ectodomain pore |
|---|---|
| Components |
|
-Supramolecule #1: murine perforin-2 (Mpeg1) ectodomain pore
| Supramolecule | Name: murine perforin-2 (Mpeg1) ectodomain pore / type: complex / Chimera: Yes / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 3.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
| Details | purified perforin-2 ecto domain protein was added to liposomes at pH3.8 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 123.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 6.0 µm / Nominal defocus min: 3.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 18.0 Å / Resolution method: FSC 0.143 CUT-OFF / Software: (Name: emClarity, RELION) / Number subtomograms used: 992 |
|---|---|
| Extraction | Number tomograms: 141 / Number images used: 1369 |
| Final angle assignment | Type: OTHER / Details: cross correlation |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)