+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-1504 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structural basis for the regulated protease and chaperone function of DegP | |||||||||
 マップデータ マップデータ | negative stain EM structure of the DegP24-OMP complex | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | protease-chaperone / electron microscopy / single particle analysis | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報peptidase Do / response to temperature stimulus / protein quality control for misfolded or incompletely synthesized proteins / : / serine-type peptidase activity / protein folding / peptidase activity / outer membrane-bounded periplasmic space / response to heat / response to oxidative stress ...peptidase Do / response to temperature stimulus / protein quality control for misfolded or incompletely synthesized proteins / : / serine-type peptidase activity / protein folding / peptidase activity / outer membrane-bounded periplasmic space / response to heat / response to oxidative stress / periplasmic space / serine-type endopeptidase activity / proteolysis / identical protein binding / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
| 手法 | 単粒子再構成法 / ネガティブ染色法 / 解像度: 23.0 Å | |||||||||
 データ登録者 データ登録者 | Krojer T / Sawa J / Schaefer E / Saibil HR / Ehrmann M / Clausen T | |||||||||
 引用 引用 |  ジャーナル: Nature / 年: 2008 ジャーナル: Nature / 年: 2008タイトル: Structural basis for the regulated protease and chaperone function of DegP. 著者: Tobias Krojer / Justyna Sawa / Eva Schäfer / Helen R Saibil / Michael Ehrmann / Tim Clausen /  要旨: All organisms have to monitor the folding state of cellular proteins precisely. The heat-shock protein DegP is a protein quality control factor in the bacterial envelope that is involved in ...All organisms have to monitor the folding state of cellular proteins precisely. The heat-shock protein DegP is a protein quality control factor in the bacterial envelope that is involved in eliminating misfolded proteins and in the biogenesis of outer-membrane proteins. Here we describe the molecular mechanisms underlying the regulated protease and chaperone function of DegP from Escherichia coli. We show that binding of misfolded proteins transforms hexameric DegP into large, catalytically active 12-meric and 24-meric multimers. A structural analysis of these particles revealed that DegP represents a protein packaging device whose central compartment is adaptable to the size and concentration of substrate. Moreover, the inner cavity serves antagonistic functions. Whereas the encapsulation of folded protomers of outer-membrane proteins is protective and might allow safe transit through the periplasm, misfolded proteins are eliminated in the molecular reaction chamber. Oligomer reassembly and concomitant activation on substrate binding may also be critical in regulating other HtrA proteases implicated in protein-folding diseases. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_1504.map.gz emd_1504.map.gz | 7.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-1504-v30.xml emd-1504-v30.xml emd-1504.xml emd-1504.xml | 6.9 KB 6.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  1504.gif 1504.gif | 77.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1504 http://ftp.pdbj.org/pub/emdb/structures/EMD-1504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1504 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_1504_validation.pdf.gz emd_1504_validation.pdf.gz | 228 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_1504_full_validation.pdf.gz emd_1504_full_validation.pdf.gz | 227.2 KB | 表示 | |
| XML形式データ |  emd_1504_validation.xml.gz emd_1504_validation.xml.gz | 5.3 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1504 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1504 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1504 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1504 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_1504.map.gz / 形式: CCP4 / 大きさ: 8.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_1504.map.gz / 形式: CCP4 / 大きさ: 8.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | negative stain EM structure of the DegP24-OMP complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 4.44 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
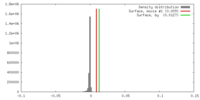
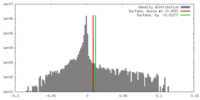
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : proteolytically inactive DegP24mer with bound Omp protein
| 全体 | 名称: proteolytically inactive DegP24mer with bound Omp protein |
|---|---|
| 要素 |
|
-超分子 #1000: proteolytically inactive DegP24mer with bound Omp protein
| 超分子 | 名称: proteolytically inactive DegP24mer with bound Omp protein タイプ: sample / ID: 1000 / 詳細: monodisperse sample / 集合状態: 24mer / Number unique components: 2 |
|---|---|
| 分子量 | 実験値: 1.13 MDa |
-分子 #1: protease
| 分子 | 名称: protease / タイプ: protein_or_peptide / ID: 1 / Name.synonym: DegP 詳細: proteolytically inactive DegP24mer with bound Omp protein コピー数: 1 / 集合状態: 24 / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  |
-実験情報
-構造解析
| 手法 | ネガティブ染色法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 0.0064 mg/mL |
|---|---|
| 染色 | タイプ: NEGATIVE 詳細: negatively stained with 2% (w/v) uranyl acetate on glow discharged, carbon-coated grids |
| 凍結 | 凍結剤: NONE / 装置: OTHER |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: OTHER / 撮影モード: OTHER |
| 試料ステージ | 試料ホルダー: single tilt / 試料ホルダーモデル: OTHER |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
- 画像解析
画像解析
| 最終 再構成 | 想定した対称性 - 点群: O (正8面体型対称) / 解像度のタイプ: BY AUTHOR / 解像度: 23.0 Å / 解像度の算出法: FSC 0.5 CUT-OFF / 詳細: octahedral symmetry was used for the reconstruction |
|---|
 ムービー
ムービー コントローラー
コントローラー












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)