[English] 日本語
 Yorodumi
Yorodumi- EMDB-14433: Structure of the Escherichia coli formate hydrogenlyase complex (... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Escherichia coli formate hydrogenlyase complex (aerobic preparation, focused refinement peripheral arm) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | FHL / group-4 membrane bound hydrogenase / [NiFe] hydrogenase / MEMBRANE PROTEIN | |||||||||
| Biological species |  | |||||||||
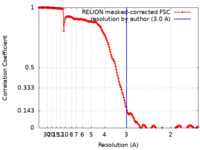
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Steinhilper R / Murphy BJ | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structure of the membrane-bound formate hydrogenlyase complex from Escherichia coli. Authors: Ralf Steinhilper / Gabriele Höff / Johann Heider / Bonnie J Murphy /  Abstract: The prototypical hydrogen-producing enzyme, the membrane-bound formate hydrogenlyase (FHL) complex from Escherichia coli, links formate oxidation at a molybdopterin-containing formate dehydrogenase ...The prototypical hydrogen-producing enzyme, the membrane-bound formate hydrogenlyase (FHL) complex from Escherichia coli, links formate oxidation at a molybdopterin-containing formate dehydrogenase to proton reduction at a [NiFe] hydrogenase. It is of intense interest due to its ability to efficiently produce H during fermentation, its reversibility, allowing H-dependent CO reduction, and its evolutionary link to respiratory complex I. FHL has been studied for over a century, but its atomic structure remains unknown. Here we report cryo-EM structures of FHL in its aerobically and anaerobically isolated forms at resolutions reaching 2.6 Å. This includes well-resolved density for conserved loops linking the soluble and membrane arms believed to be essential in coupling enzymatic turnover to ion translocation across the membrane in the complex I superfamily. We evaluate possible structural determinants of the bias toward hydrogen production over its oxidation and describe an unpredicted metal-binding site near the interface of FdhF and HycF subunits that may play a role in redox-dependent regulation of FdhF interaction with the complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14433.map.gz emd_14433.map.gz | 396 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14433-v30.xml emd-14433-v30.xml emd-14433.xml emd-14433.xml | 13.2 KB 13.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14433_fsc.xml emd_14433_fsc.xml | 17 KB | Display |  FSC data file FSC data file |
| Images |  emd_14433.png emd_14433.png | 61.6 KB | ||
| Masks |  emd_14433_msk_1.map emd_14433_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-14433.cif.gz emd-14433.cif.gz | 3.7 KB | ||
| Others |  emd_14433_half_map_1.map.gz emd_14433_half_map_1.map.gz emd_14433_half_map_2.map.gz emd_14433_half_map_2.map.gz | 338.5 MB 338.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14433 http://ftp.pdbj.org/pub/emdb/structures/EMD-14433 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14433 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14433 | HTTPS FTP |
-Validation report
| Summary document |  emd_14433_validation.pdf.gz emd_14433_validation.pdf.gz | 978.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14433_full_validation.pdf.gz emd_14433_full_validation.pdf.gz | 977.9 KB | Display | |
| Data in XML |  emd_14433_validation.xml.gz emd_14433_validation.xml.gz | 24.1 KB | Display | |
| Data in CIF |  emd_14433_validation.cif.gz emd_14433_validation.cif.gz | 31.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14433 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14433 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14433 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14433 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14433.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14433.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
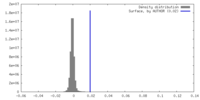
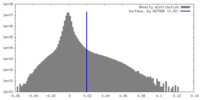
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_14433_msk_1.map emd_14433_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_14433_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_14433_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Escherichia coli formate hydrogenlyase complex
| Entire | Name: Escherichia coli formate hydrogenlyase complex |
|---|---|
| Components |
|
-Supramolecule #1: Escherichia coli formate hydrogenlyase complex
| Supramolecule | Name: Escherichia coli formate hydrogenlyase complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.6 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)