[English] 日本語
 Yorodumi
Yorodumi- EMDB-14430: Structure of the Escherichia coli formate hydrogenlyase complex (... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Escherichia coli formate hydrogenlyase complex (aerobic preparation, composite structure) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | FHL / group-4 membrane bound hydrogenase / [NiFe] hydrogenase / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationformate dehydrogenase (hydrogenase) / formate oxidation / oxidoreductase activity, acting on the aldehyde or oxo group of donors / formate dehydrogenase complex / glucose catabolic process / formate dehydrogenase (NAD+) activity / anaerobic electron transport chain / urate catabolic process / molybdopterin cofactor binding / anaerobic respiration ...formate dehydrogenase (hydrogenase) / formate oxidation / oxidoreductase activity, acting on the aldehyde or oxo group of donors / formate dehydrogenase complex / glucose catabolic process / formate dehydrogenase (NAD+) activity / anaerobic electron transport chain / urate catabolic process / molybdopterin cofactor binding / anaerobic respiration / oxidoreductase activity, acting on NAD(P)H / nickel cation binding / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / aerobic respiration / respiratory electron transport chain / NAD binding / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / metal ion binding / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Steinhilper R / Murphy BJ | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structure of the membrane-bound formate hydrogenlyase complex from Escherichia coli. Authors: Ralf Steinhilper / Gabriele Höff / Johann Heider / Bonnie J Murphy /  Abstract: The prototypical hydrogen-producing enzyme, the membrane-bound formate hydrogenlyase (FHL) complex from Escherichia coli, links formate oxidation at a molybdopterin-containing formate dehydrogenase ...The prototypical hydrogen-producing enzyme, the membrane-bound formate hydrogenlyase (FHL) complex from Escherichia coli, links formate oxidation at a molybdopterin-containing formate dehydrogenase to proton reduction at a [NiFe] hydrogenase. It is of intense interest due to its ability to efficiently produce H during fermentation, its reversibility, allowing H-dependent CO reduction, and its evolutionary link to respiratory complex I. FHL has been studied for over a century, but its atomic structure remains unknown. Here we report cryo-EM structures of FHL in its aerobically and anaerobically isolated forms at resolutions reaching 2.6 Å. This includes well-resolved density for conserved loops linking the soluble and membrane arms believed to be essential in coupling enzymatic turnover to ion translocation across the membrane in the complex I superfamily. We evaluate possible structural determinants of the bias toward hydrogen production over its oxidation and describe an unpredicted metal-binding site near the interface of FdhF and HycF subunits that may play a role in redox-dependent regulation of FdhF interaction with the complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14430.map.gz emd_14430.map.gz | 394.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14430-v30.xml emd-14430-v30.xml emd-14430.xml emd-14430.xml | 20 KB 20 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14430.png emd_14430.png | 93.6 KB | ||
| Filedesc metadata |  emd-14430.cif.gz emd-14430.cif.gz | 7.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14430 http://ftp.pdbj.org/pub/emdb/structures/EMD-14430 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14430 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14430 | HTTPS FTP |
-Validation report
| Summary document |  emd_14430_validation.pdf.gz emd_14430_validation.pdf.gz | 584.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14430_full_validation.pdf.gz emd_14430_full_validation.pdf.gz | 584.4 KB | Display | |
| Data in XML |  emd_14430_validation.xml.gz emd_14430_validation.xml.gz | 8 KB | Display | |
| Data in CIF |  emd_14430_validation.cif.gz emd_14430_validation.cif.gz | 9.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14430 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14430 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14430 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14430 | HTTPS FTP |
-Related structure data
| Related structure data |  7z0tMC  7z0sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14430.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14430.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
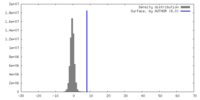
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Escherichia coli formate hydrogenlyase complex
+Supramolecule #1: Escherichia coli formate hydrogenlyase complex
+Macromolecule #1: Formate hydrogenlyase subunit 3
+Macromolecule #2: Formate hydrogenlyase subunit 5
+Macromolecule #3: Formate hydrogenlyase subunit 2
+Macromolecule #4: Formate hydrogenlyase subunit 7
+Macromolecule #5: Formate hydrogenlyase subunit 6
+Macromolecule #6: Formate hydrogenlyase subunit 4
+Macromolecule #7: Formate dehydrogenase H
+Macromolecule #8: NICKEL (II) ION
+Macromolecule #9: CARBONMONOXIDE-(DICYANO) IRON
+Macromolecule #10: IRON/SULFUR CLUSTER
+Macromolecule #11: FE (III) ION
+Macromolecule #12: 2-AMINO-5,6-DIMERCAPTO-7-METHYL-3,7,8A,9-TETRAHYDRO-8-OXA-1,3,9,1...
+Macromolecule #13: MOLYBDENUM(VI) ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: C-flat-2/1 / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.6 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: OTHER / Software - Name: RELION (ver. 3) Details: This is a composite map. The maps that constitute this composite map have resolutions between 3.0 and 3.4 A. These have all been determined by FSC 0.143 cut-off. Number images used: 90459 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: RELION (ver. 3) |
 Movie
Movie Controller
Controller


















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)