+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | phospho-STING binding to adaptor protein complex-1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | STING / innate immunity / TGN / AP-1 / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationbasolateral protein secretion / Lysosome Vesicle Biogenesis / endosome to melanosome transport / AP-1 adaptor complex / mitotic cleavage furrow ingression / trans-Golgi Network Vesicle Budding / platelet dense granule organization / Glycosphingolipid transport / melanosome assembly / regulation of receptor internalization ...basolateral protein secretion / Lysosome Vesicle Biogenesis / endosome to melanosome transport / AP-1 adaptor complex / mitotic cleavage furrow ingression / trans-Golgi Network Vesicle Budding / platelet dense granule organization / Glycosphingolipid transport / melanosome assembly / regulation of receptor internalization / Intra-Golgi traffic / STING complex / regulation of Arp2/3 complex-mediated actin nucleation / STAT6-mediated induction of chemokines / Golgi Associated Vesicle Biogenesis / Synthesis of PIPs at the Golgi membrane / protein localization to endoplasmic reticulum / clathrin adaptor activity / 2',3'-cyclic GMP-AMP binding / cyclic-di-GMP binding / MHC class II antigen presentation / STING mediated induction of host immune responses / serine/threonine protein kinase complex / Nef Mediated CD4 Down-regulation / positive regulation of type I interferon-mediated signaling pathway / IRF3-mediated induction of type I IFN / dendritic spine organization / cGAS/STING signaling pathway / long-term synaptic depression / determination of left/right symmetry / proton channel activity / clathrin-coated vesicle / reticulophagy / pattern recognition receptor signaling pathway / COPI-dependent Golgi-to-ER retrograde traffic / clathrin binding / Lysosome Vesicle Biogenesis / cytoplasmic pattern recognition receptor signaling pathway / Golgi Associated Vesicle Biogenesis / cellular response to exogenous dsRNA / Synthesis of PIPs at the plasma membrane / cell leading edge / protein complex oligomerization / autophagosome membrane / positive regulation of macroautophagy / intracellular copper ion homeostasis / autophagosome assembly / positive regulation of type I interferon production / protein targeting / : / cellular response to interferon-beta / COPI-mediated anterograde transport / vesicle-mediated transport / positive regulation of defense response to virus by host / clathrin-coated pit / Neutrophil degranulation / endoplasmic reticulum-Golgi intermediate compartment membrane / signaling adaptor activity / MHC class II antigen presentation / antiviral innate immune response / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / activation of innate immune response / protein serine/threonine kinase binding / autophagosome / positive regulation of interferon-beta production / Regulation of innate immune responses to cytosolic DNA / secretory granule membrane / cytoplasmic vesicle membrane / Nef mediated downregulation of MHC class I complex cell surface expression / sarcomere / trans-Golgi network membrane / small monomeric GTPase / intracellular protein transport / kidney development / trans-Golgi network / cellular response to virus / synaptic vesicle / SARS-CoV-1 activates/modulates innate immune responses / peroxisome / presynapse / heart development / regulation of inflammatory response / defense response to virus / DNA-binding transcription factor binding / RNA polymerase II-specific DNA-binding transcription factor binding / early endosome / mitochondrial outer membrane / endosome / neuron projection / postsynaptic density / cilium / ciliary basal body / protein domain specific binding / Golgi membrane / innate immune response / lysosomal membrane / focal adhesion / intracellular membrane-bounded organelle / GTPase activity / ubiquitin protein ligase binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
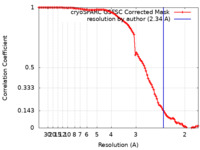
| Method | single particle reconstruction / cryo EM / Resolution: 2.34 Å | |||||||||
 Authors Authors | Xu P / Ablasser A | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Clathrin-associated AP-1 controls termination of STING signalling. Authors: Ying Liu / Pengbiao Xu / Sophie Rivara / Chong Liu / Jonathan Ricci / Xuefeng Ren / James H Hurley / Andrea Ablasser /   Abstract: Stimulator of interferon genes (STING) functions downstream of cyclic GMP-AMP synthase in DNA sensing or as a direct receptor for bacterial cyclic dinucleotides and small molecules to activate ...Stimulator of interferon genes (STING) functions downstream of cyclic GMP-AMP synthase in DNA sensing or as a direct receptor for bacterial cyclic dinucleotides and small molecules to activate immunity during infection, cancer and immunotherapy. Precise regulation of STING is essential to ensure balanced immune responses and prevent detrimental autoinflammation. After activation, STING, a transmembrane protein, traffics from the endoplasmic reticulum to the Golgi, where its phosphorylation by the protein kinase TBK1 enables signal transduction. The mechanism that ends STING signalling at the Golgi remains unknown. Here we show that adaptor protein complex 1 (AP-1) controls the termination of STING-dependent immune activation. We find that AP-1 sorts phosphorylated STING into clathrin-coated transport vesicles for delivery to the endolysosomal system, where STING is degraded. We identify a highly conserved dileucine motif in the cytosolic C-terminal tail (CTT) of STING that, together with TBK1-dependent CTT phosphorylation, dictates the AP-1 engagement of STING. A cryo-electron microscopy structure of AP-1 in complex with phosphorylated STING explains the enhanced recognition of TBK1-activated STING. We show that suppression of AP-1 exacerbates STING-induced immune responses. Our results reveal a structural mechanism of negative regulation of STING and establish that the initiation of signalling is inextricably associated with its termination to enable transient activation of immunity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14312.map.gz emd_14312.map.gz | 51.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14312-v30.xml emd-14312-v30.xml emd-14312.xml emd-14312.xml | 26.8 KB 26.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14312_fsc.xml emd_14312_fsc.xml | 9.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_14312.png emd_14312.png | 119.2 KB | ||
| Filedesc metadata |  emd-14312.cif.gz emd-14312.cif.gz | 7.9 KB | ||
| Others |  emd_14312_half_map_1.map.gz emd_14312_half_map_1.map.gz emd_14312_half_map_2.map.gz emd_14312_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14312 http://ftp.pdbj.org/pub/emdb/structures/EMD-14312 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14312 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14312 | HTTPS FTP |
-Validation report
| Summary document |  emd_14312_validation.pdf.gz emd_14312_validation.pdf.gz | 912.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14312_full_validation.pdf.gz emd_14312_full_validation.pdf.gz | 911.8 KB | Display | |
| Data in XML |  emd_14312_validation.xml.gz emd_14312_validation.xml.gz | 17.5 KB | Display | |
| Data in CIF |  emd_14312_validation.cif.gz emd_14312_validation.cif.gz | 22.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14312 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14312 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14312 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14312 | HTTPS FTP |
-Related structure data
| Related structure data |  7r4hMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14312.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14312.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_14312_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
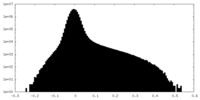
| Projections & Slices |
| ||||||||||||
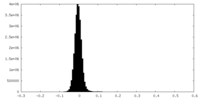
| Density Histograms |
-Half map: #1
| File | emd_14312_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
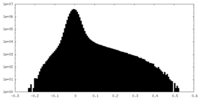
| Projections & Slices |
| ||||||||||||
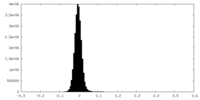
| Density Histograms |
- Sample components
Sample components
-Entire : phospho-STING binding to adaptor protein complex-1
| Entire | Name: phospho-STING binding to adaptor protein complex-1 |
|---|---|
| Components |
|
-Supramolecule #1: phospho-STING binding to adaptor protein complex-1
| Supramolecule | Name: phospho-STING binding to adaptor protein complex-1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: AP-1 complex subunit beta-1
| Macromolecule | Name: AP-1 complex subunit beta-1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.008422 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTDSKYFTTT KKGEIFELKA ELNSDKKEKK KEAVKKVIAS MTVGKDVSAL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN TFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQLVEDQ G FLDTLKDL ...String: MTDSKYFTTT KKGEIFELKA ELNSDKKEKK KEAVKKVIAS MTVGKDVSAL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN TFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQLVEDQ G FLDTLKDL ISDSNPMVVA NAVAALSEIA ESHPSSNLLD LNPQSINKLL TALNECTEWG QIFILDCLAN YMPKDDREAQ SI CERVTPR LSHANSAVVL SAVKVLMKFM EMLSKDLDYY GTLLKKLAPP LVTLLSAEPE LQYVALRNIN LIVQKRPEIL KHE MKVFFV KYNDPIYVKL EKLDIMIRLA SQANIAQVLA ELREYATEVD VDFVRKAVRA IGRCAIKVEQ SAERCVSTLL DLIQ TKVNY VVQEAIVVIK DIFRKYPNKY ESVIATLCEN LDSLDEPEAR AAMIWIVGEY AERIDNADEL LESFLEGFHD KSTQV QLQL LTAIVKLFLK KPTETQELVQ QVLSLATQDS DNPDLRDRGY IYWRLLSTDP VAAKEVVLAE KPLISEETDL IEPTLL DEL ICYIGTLASV YHKPPSAFVE G UniProtKB: AP-1 complex subunit beta-1 |
-Macromolecule #2: ADP-ribosylation factor 1
| Macromolecule | Name: ADP-ribosylation factor 1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.9366 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EMRILMVGLD AAGKTTILYK LKLGEIVTTI PTIGFNVETV EYKNISFTVW DVGGLDKIRP LWRHYFQNTQ GLIFVVDSND RERVNEARE ELMRMLAEDE LRDAVLLVFA NKQDLPNAMN AAEITDKLGL HSLRHRNWYI QATCATSGDG LYEGLDWLSN Q LRNQK UniProtKB: ADP-ribosylation factor 1 |
-Macromolecule #3: AP-1 complex subunit gamma-1
| Macromolecule | Name: AP-1 complex subunit gamma-1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 67.399242 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPAPIRLREL IRTIRTARTQ AEEREMIQKE CAAIRSSFRE EDNTYRCRNV AKLLYMHMLG YPAHFGQLEC LKLIASQKFT DKRIGYLGA MLLLDERQDV HLLMTNCIKN DLNHSTQFVQ GLALCTLGCM GSSEMCRDLA GEVEKLLKTS NSYLRKKAAL C AVHVIRKV ...String: MPAPIRLREL IRTIRTARTQ AEEREMIQKE CAAIRSSFRE EDNTYRCRNV AKLLYMHMLG YPAHFGQLEC LKLIASQKFT DKRIGYLGA MLLLDERQDV HLLMTNCIKN DLNHSTQFVQ GLALCTLGCM GSSEMCRDLA GEVEKLLKTS NSYLRKKAAL C AVHVIRKV PELMEMFLPA TKNLLNEKNH GVLHTSVVLL TEMCERSPDM LAHFRKLVPQ LVRILKNLIM SGYSPEHDVS GI SDPFLQV RILRLLRILG RNDDDSSEAM NDILAQVATN TETSKNVGNA ILYETVLTIM DIKSESGLRV LAINILGRFL LNN DKNIRY VALTSLLKTV QTDHNAVQRH RSTIVDCLKD LDVSIKRRAM ELSFALVNGN NIRGMMKELL YFLDSCEPEF KADC ASGIF LAAEKYAPSK RWHIDTIMRV LTTAGSYVRD DAVPNLIQLI TNSVEMHAYT VQRLYKAILG DYSQQPLVQV AAWCI GEYG DLLVSGQCEE EEPIQVTEDE VLDILESVLI SNMSTSVTRG YALTAIMKLS TRFTCTVNRI KKVVSIYGSS IDVELQ QRA VEYNALFKKY DHMRSALLER MPVMEKVTTN GP UniProtKB: AP-1 complex subunit gamma-1 |
-Macromolecule #4: Stimulator of interferon genes protein
| Macromolecule | Name: Stimulator of interferon genes protein / type: protein_or_peptide / ID: 4 / Details: phospho-STING tail / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.065068 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QEPELLI(SEP)G UniProtKB: Stimulator of interferon genes protein |
-Macromolecule #5: AP-1 complex subunit mu-1
| Macromolecule | Name: AP-1 complex subunit mu-1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.60673 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSASAVYVLD LKGKVLICRN YRGDVDMSEV EHFMPILMEK EEEGMLSPIL AHGGVRFMWI KHNNLYLVAT SKKNACVSLV FSFLYKVVQ VFSEYFKELE EESIRDNFVI IYELLDELMD FGYPQTTDSK ILQEYITQEG HKLETGAPRP PATVTNAVSW R SEGIKYRK ...String: MSASAVYVLD LKGKVLICRN YRGDVDMSEV EHFMPILMEK EEEGMLSPIL AHGGVRFMWI KHNNLYLVAT SKKNACVSLV FSFLYKVVQ VFSEYFKELE EESIRDNFVI IYELLDELMD FGYPQTTDSK ILQEYITQEG HKLETGAPRP PATVTNAVSW R SEGIKYRK NEVFLDVIEA VNLLVSANGN VLRSEIVGSI KMRVFLSGMP ELRLGLNDKV LFDNTGRGKS KSVELEDVKF HQ CVRLSRF ENDRTISFIP PDGEFELMSY RLNTHVKPLI WIESVIEKHS HSRIEYMVKA KSQFKRRSTA NNVEIHIPVP NDA DSPKFK TTVGSVKWVP ENSEIVWSVK SFPGGKEYLM RAHFGLPSVE AEDKEGKPPI SVKFEIPYFT TSGIQVRYLK IIEK SGYQA LPWVRYITQN GDYQLRTQ UniProtKB: AP-1 complex subunit mu-1 |
-Macromolecule #6: AP-1 complex subunit sigma-3
| Macromolecule | Name: AP-1 complex subunit sigma-3 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.321338 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIHFILLFSR QGKLRLQKWY ITLPDKERKK ITREIVQIIL SRGHRTSSFV DWKELKLVYK RYASLYFCCA IENQDNELLT LEIVHRYVE LLDKYFGNVC ELDIIFNFEK AYFILDEFII GGEIQETSKK IAVKAIEDSD MLQEVSTVCQ TMGER UniProtKB: AP-1 complex subunit sigma-3 |
-Macromolecule #7: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 7 / Number of copies: 2 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #8: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 8 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: PBS buffer | |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)