[English] 日本語
 Yorodumi
Yorodumi- EMDB-14192: Structure of the ribosome-nascent chain containing an ER signal s... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-14192 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the ribosome-nascent chain containing an ER signal sequence in complex with NAC | |||||||||||||||
 Map data Map data | Ribosome nascent chain containing an ER signal sequence in complex with NAC | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Ribosome / SRP / NAC / nascent chain / co-translational / Endoplasmic reticulum / co-translational protein targeting / co-translational folding | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of striated muscle cell apoptotic process / regulation of skeletal muscle fiber development / positive regulation of cell proliferation involved in heart morphogenesis / Major pathway of rRNA processing in the nucleolus and cytosol / GTP hydrolysis and joining of the 60S ribosomal subunit / positive regulation of skeletal muscle tissue growth / L13a-mediated translational silencing of Ceruloplasmin expression / SRP-dependent cotranslational protein targeting to membrane / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) ...negative regulation of striated muscle cell apoptotic process / regulation of skeletal muscle fiber development / positive regulation of cell proliferation involved in heart morphogenesis / Major pathway of rRNA processing in the nucleolus and cytosol / GTP hydrolysis and joining of the 60S ribosomal subunit / positive regulation of skeletal muscle tissue growth / L13a-mediated translational silencing of Ceruloplasmin expression / SRP-dependent cotranslational protein targeting to membrane / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / negative regulation of protein localization to endoplasmic reticulum / nascent polypeptide-associated complex / cardiac ventricle development / heart trabecula morphogenesis / skeletal muscle tissue regeneration / ribosomal subunit / ubiquitin ligase inhibitor activity / positive regulation of signal transduction by p53 class mediator / rough endoplasmic reticulum / MDM2/MDM4 family protein binding / cytosolic ribosome / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / wound healing / antimicrobial humoral immune response mediated by antimicrobial peptide / protein transport / regulation of translation / large ribosomal subunit / ribosomal large subunit assembly / 5S rRNA binding / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / killing of cells of another organism / in utero embryonic development / defense response to Gram-negative bacterium / cytoplasmic translation / transcription coactivator activity / tRNA binding / rRNA binding / postsynaptic density / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / synapse / nucleolus / negative regulation of transcription by RNA polymerase II / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / DNA binding / RNA binding / extracellular exosome / zinc ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||||||||
 Authors Authors | Jomaa A / Gamerdinger M | |||||||||||||||
| Funding support |  Switzerland, Switzerland,  United Kingdom, United Kingdom,  Germany, Germany,  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Mechanism of signal sequence handover from NAC to SRP on ribosomes during ER-protein targeting. Authors: Ahmad Jomaa / Martin Gamerdinger / Hao-Hsuan Hsieh / Annalena Wallisch / Viswanathan Chandrasekaran / Zeynel Ulusoy / Alain Scaiola / Ramanujan S Hegde / Shu-Ou Shan / Nenad Ban / Elke Deuerling /     Abstract: The nascent polypeptide-associated complex (NAC) interacts with newly synthesized proteins at the ribosomal tunnel exit and competes with the signal recognition particle (SRP) to prevent mistargeting ...The nascent polypeptide-associated complex (NAC) interacts with newly synthesized proteins at the ribosomal tunnel exit and competes with the signal recognition particle (SRP) to prevent mistargeting of cytosolic and mitochondrial polypeptides to the endoplasmic reticulum (ER). How NAC antagonizes SRP and how this is overcome by ER targeting signals are unknown. Here, we found that NAC uses two domains with opposing effects to control SRP access. The core globular domain prevented SRP from binding to signal-less ribosomes, whereas a flexibly attached domain transiently captured SRP to permit scanning of nascent chains. The emergence of an ER-targeting signal destabilized NAC's globular domain and facilitated SRP access to the nascent chain. These findings elucidate how NAC hands over the signal sequence to SRP and imparts specificity of protein localization. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14192.map.gz emd_14192.map.gz | 321.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14192-v30.xml emd-14192-v30.xml emd-14192.xml emd-14192.xml | 72.7 KB 72.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14192.png emd_14192.png | 157.8 KB | ||
| Filedesc metadata |  emd-14192.cif.gz emd-14192.cif.gz | 15.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14192 http://ftp.pdbj.org/pub/emdb/structures/EMD-14192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14192 | HTTPS FTP |
-Related structure data
| Related structure data |  7qwrMC  7qwqC  7qwsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14192.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14192.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ribosome nascent chain containing an ER signal sequence in complex with NAC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
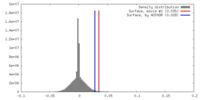
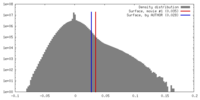
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : ribosome-nascent chain containing an ER signal sequence in comple...
+Supramolecule #1: ribosome-nascent chain containing an ER signal sequence in comple...
+Supramolecule #2: Ribosome
+Supramolecule #3: Nascent chain pre-prolactin
+Supramolecule #4: Nascent polypeptide-associated complex subunit alpha
+Supramolecule #5: Isoform 2 of Transcription factor BTF3
+Macromolecule #1: Nascent chain pre-prolactin
+Macromolecule #2: Nascent polypeptide-associated complex subunit alpha
+Macromolecule #3: Isoform 2 of Transcription factor BTF3
+Macromolecule #4: 60S ribosomal protein L8
+Macromolecule #5: 60S ribosomal protein L29
+Macromolecule #6: Ribosomal protein L3
+Macromolecule #7: 60S ribosomal protein L30
+Macromolecule #8: 60S ribosomal protein L4
+Macromolecule #9: Ribosomal protein L31
+Macromolecule #10: Ribosomal_L18_c domain-containing protein
+Macromolecule #11: Ribosomal protein L32
+Macromolecule #12: 60S ribosomal protein L6
+Macromolecule #13: 60S ribosomal protein L35a
+Macromolecule #14: uL30
+Macromolecule #15: 60S ribosomal protein L34
+Macromolecule #16: 60S ribosomal protein L7a
+Macromolecule #17: 60S ribosomal protein L35
+Macromolecule #18: 60S ribosomal protein L9
+Macromolecule #19: 60S ribosomal protein L36
+Macromolecule #20: 60S ribosomal protein L10
+Macromolecule #21: Ribosomal protein L37
+Macromolecule #22: Ribosomal protein L11
+Macromolecule #23: 60S ribosomal protein L38
+Macromolecule #24: L13
+Macromolecule #25: Ribosomal protein L39
+Macromolecule #26: 60S ribosomal protein L14
+Macromolecule #27: 60S ribosomal protein L40
+Macromolecule #28: Ribosomal protein L15
+Macromolecule #29: 60s ribosomal protein l41
+Macromolecule #30: 60S ribosomal protein L13a
+Macromolecule #31: eL42
+Macromolecule #32: 60S ribosomal protein L17
+Macromolecule #33: eL43
+Macromolecule #34: eL18
+Macromolecule #35: 60S ribosomal protein L28
+Macromolecule #36: 60S ribosomal protein L19
+Macromolecule #37: 60S ribosomal protein L18a
+Macromolecule #38: 60S ribosomal protein L21
+Macromolecule #39: 60S ribosomal protein L22
+Macromolecule #40: Ribosomal protein L23
+Macromolecule #41: Ribosomal protein L24
+Macromolecule #43: Ribosomal_L23eN domain-containing protein
+Macromolecule #45: Ribosomal protein L26
+Macromolecule #47: 60S ribosomal protein L27
+Macromolecule #48: 60S ribosomal protein L27a
+Macromolecule #42: 28S rRNA
+Macromolecule #44: 5S ribosomal RNA
+Macromolecule #46: 5.8S ribosomal RNA
+Macromolecule #49: ZINC ION
+Macromolecule #50: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 95 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 2.9 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.1) / Number images used: 44040 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)