[English] 日本語
 Yorodumi
Yorodumi- EMDB-13385: Low resolution Cryo-EM structure of full-length insulin receptor ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13385 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Low resolution Cryo-EM structure of full-length insulin receptor bound to 3 insulin with visible ddm micelle, conf 1 | |||||||||
 Map data Map data | DDM solubilised full-length human insulin receptor with three insulins bound with visible detergent micelle. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Insulin / Receptor / Complex / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of female gonad development / positive regulation of meiotic cell cycle / insulin-like growth factor II binding / positive regulation of developmental growth / male sex determination / insulin receptor complex / insulin-like growth factor I binding / positive regulation of protein-containing complex disassembly / insulin receptor activity / exocrine pancreas development ...regulation of female gonad development / positive regulation of meiotic cell cycle / insulin-like growth factor II binding / positive regulation of developmental growth / male sex determination / insulin receptor complex / insulin-like growth factor I binding / positive regulation of protein-containing complex disassembly / insulin receptor activity / exocrine pancreas development / dendritic spine maintenance / cargo receptor activity / insulin binding / adrenal gland development / neuronal cell body membrane / PTB domain binding / negative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / regulation of protein secretion / Insulin processing / positive regulation of peptide hormone secretion / positive regulation of respiratory burst / amyloid-beta clearance / negative regulation of acute inflammatory response / Regulation of gene expression in beta cells / alpha-beta T cell activation / positive regulation of receptor internalization / regulation of embryonic development / insulin receptor substrate binding / positive regulation of dendritic spine maintenance / Synthesis, secretion, and deacylation of Ghrelin / negative regulation of respiratory burst involved in inflammatory response / protein kinase activator activity / epidermis development / activation of protein kinase B activity / negative regulation of protein secretion / negative regulation of gluconeogenesis / positive regulation of insulin receptor signaling pathway / positive regulation of glycogen biosynthetic process / fatty acid homeostasis / Signal attenuation / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / negative regulation of lipid catabolic process / positive regulation of lipid biosynthetic process / heart morphogenesis / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / transport across blood-brain barrier / regulation of protein localization to plasma membrane / phosphatidylinositol 3-kinase binding / nitric oxide-cGMP-mediated signaling / transport vesicle / COPI-mediated anterograde transport / positive regulation of nitric-oxide synthase activity / Insulin receptor recycling / negative regulation of reactive oxygen species biosynthetic process / insulin-like growth factor receptor binding / positive regulation of brown fat cell differentiation / NPAS4 regulates expression of target genes / dendrite membrane / neuron projection maintenance / endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of mitotic nuclear division / Insulin receptor signalling cascade / receptor-mediated endocytosis / positive regulation of glycolytic process / positive regulation of cytokine production / endosome lumen / positive regulation of long-term synaptic potentiation / acute-phase response / positive regulation of protein secretion / positive regulation of D-glucose import / learning / insulin receptor binding / positive regulation of cell differentiation / Regulation of insulin secretion / wound healing / positive regulation of neuron projection development / receptor protein-tyrosine kinase / hormone activity / negative regulation of protein catabolic process / regulation of synaptic plasticity / caveola / cellular response to growth factor stimulus / receptor internalization / positive regulation of protein localization to nucleus / Golgi lumen / vasodilation / cognition / memory / male gonad development / glucose metabolic process / cellular response to insulin stimulus / positive regulation of nitric oxide biosynthetic process / insulin receptor signaling pathway / late endosome / cell-cell signaling Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
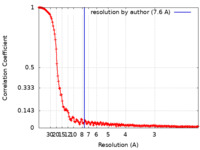
| Method | single particle reconstruction / cryo EM / Resolution: 7.6 Å | |||||||||
 Authors Authors | Nielsen JA / Slaaby R | |||||||||
| Funding support |  Denmark, 1 items Denmark, 1 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2022 Journal: J Mol Biol / Year: 2022Title: Structural Investigations of Full-Length Insulin Receptor Dynamics and Signalling. Authors: Jeppe Nielsen / Jakob Brandt / Thomas Boesen / Tina Hummelshøj / Rita Slaaby / Gerd Schluckebier / Poul Nissen /  Abstract: Insulin regulates glucose homeostasis via binding and activation of the insulin receptor dimer at two distinct pairs of binding sites 1 and 2. Here, we present cryo-EM studies of full-length human ...Insulin regulates glucose homeostasis via binding and activation of the insulin receptor dimer at two distinct pairs of binding sites 1 and 2. Here, we present cryo-EM studies of full-length human insulin receptor (hIR) in an active state obtained at non-saturating, physiologically relevant insulin conditions. Insulin binds asymmetrically to the receptor under these conditions, occupying up to three of the four possible binding sites. Deletion analysis of the receptor together with site specific peptides and insulin analogs used in binding studies show that both sites 1 and 2 are required for high insulin affinity. We identify a homotypic interaction of the fibronectin type III domain (FnIII-3) of IR resulting in tight interaction of membrane proximal domains of the active, asymmetric receptor dimer. Our results show how insulin binding at two distinct types of sites disrupts the autoinhibited apo-IR dimer and stabilizes the active dimer. We propose an insulin binding and activation mechanism, which is sequential, exhibits negative cooperativity, and is based on asymmetry at physiological insulin concentrations with one to three insulin molecules activating IR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13385.map.gz emd_13385.map.gz | 142.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13385-v30.xml emd-13385-v30.xml emd-13385.xml emd-13385.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13385_fsc.xml emd_13385_fsc.xml | 19.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_13385.png emd_13385.png | 75.9 KB | ||
| Masks |  emd_13385_msk_1.map emd_13385_msk_1.map | 282.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13385.cif.gz emd-13385.cif.gz | 6.4 KB | ||
| Others |  emd_13385_half_map_1.map.gz emd_13385_half_map_1.map.gz emd_13385_half_map_2.map.gz emd_13385_half_map_2.map.gz | 261.8 MB 261.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13385 http://ftp.pdbj.org/pub/emdb/structures/EMD-13385 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13385 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13385 | HTTPS FTP |
-Validation report
| Summary document |  emd_13385_validation.pdf.gz emd_13385_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13385_full_validation.pdf.gz emd_13385_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_13385_validation.xml.gz emd_13385_validation.xml.gz | 22.7 KB | Display | |
| Data in CIF |  emd_13385_validation.cif.gz emd_13385_validation.cif.gz | 30.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13385 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13385 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13385 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13385 | HTTPS FTP |
-Related structure data
| Related structure data |  7pg0MC  7pg2C  7pg3C  7pg4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13385.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13385.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | DDM solubilised full-length human insulin receptor with three insulins bound with visible detergent micelle. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0898 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13385_msk_1.map emd_13385_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_13385_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_13385_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : DDM solubilised full-length human insulin receptor with three ins...
| Entire | Name: DDM solubilised full-length human insulin receptor with three insulins bound |
|---|---|
| Components |
|
-Supramolecule #1: DDM solubilised full-length human insulin receptor with three ins...
| Supramolecule | Name: DDM solubilised full-length human insulin receptor with three insulins bound type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 460 KDa |
-Macromolecule #1: Isoform Short of Insulin receptor
| Macromolecule | Name: Isoform Short of Insulin receptor / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 156.697578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MATGGRRGAA AAPLLVAVAA LLLGAAGHLY PGEVCPGMDI RNNLTRLHEL ENCSVIEGHL QILLMFKTRP EDFRDLSFPK LIMITDYLL LFRVYGLESL KDLFPNLTVI RGSRLFFNYA LVIFEMVHLK ELGLYNLMNI TRGSVRIEKN NELCYLATID W SRILDSVE ...String: MATGGRRGAA AAPLLVAVAA LLLGAAGHLY PGEVCPGMDI RNNLTRLHEL ENCSVIEGHL QILLMFKTRP EDFRDLSFPK LIMITDYLL LFRVYGLESL KDLFPNLTVI RGSRLFFNYA LVIFEMVHLK ELGLYNLMNI TRGSVRIEKN NELCYLATID W SRILDSVE DNYIVLNKDD NEECGDICPG TAKGKTNCPA TVINGQFVER CWTHSHCQKV CPTICKSHGC TAEGLCCHSE CL GNCSQPD DPTKCVACRN FYLDGRCVET CPPPYYHFQD WRCVNFSFCQ DLHHKCKNSR RQGCHQYVIH NNKCIPECPS GYT MNSSNL LCTPCLGPCP KVCHLLEGEK TIDSVTSAQE LRGCTVINGS LIINIRGGNN LAAELEANLG LIEEISGYLK IRRS YALVS LSFFRKLRLI RGETLEIGNY SFYALDNQNL RQLWDWSKHN LTITQGKLFF HYNPKLCLSE IHKMEEVSGT KGRQE RNDI ALKTNGDQAS CENELLKFSY IRTSFDKILL RWEPYWPPDF RDLLGFMLFY KEAPYQNVTE FDGQDACGSN SWTVVD IDP PLRSNDPKSQ NHPGWLMRGL KPWTQYAIFV KTLVTFSDER RTYGAKSDII YVQTDATNPS VPLDPISVSN SSSQIIL KW KPPSDPNGNI THYLVFWERQ AEDSELFELD YCLKGLKLPS RTWSPPFESE DSQKHNQSEY EDSAGECCSC PKTDSQIL K ELEESSFRKT FEDYLHNVVF VPRPSRKRRS LGDVGNVTVA VPTVAAFPNT SSTSVPTSPE EHRPFEKVVN KESLVISGL RHFTGYRIEL QACNQDTPEE RCSVAAYVSA RTMPEAKADD IVGPVTHEIF ENNVVHLMWQ EPKEPNGLIV LYEVSYRRYG DEELHLCVS RKHFALERGC RLRGLSPGNY SVRIRATSLA GNGSWTEPTY FYVTDYLDVP SNIAKIIIGP LIFVFLFSVV I GSIYLFLR KRQPDGPLGP LYASSNPEYL SASDVFPCSV YVPDEWEVSR EKITLLRELG QGSFGMVYEG NARDIIKGEA ET RVAVKTV NESASLRERI EFLNEASVMK GFTCHHVVRL LGVVSKGQPT LVVMELMAHG DLKSYLRSLR PEAENNPGRP PPT LQEMIQ MAAEIADGMA YLNAKKFVHR DLAARNCMVA HDFTVKIGDF GMTRDIYETD YYRKGGKGLL PVRWMAPESL KDGV FTTSS DMWSFGVVLW EITSLAEQPY QGLSNEQVLK FVMDGGYLDQ PDNCPERVTD LMRMCWQFNP KMRPTFLEIV NLLKD DLHP SFPEVSFFHS EENKAPESEE LEMEFEDMEN VPLDRSSHCQ REEAGGRDGG SSLGFKRSYE EHIPYTHMNG GKKNGR ILT LPRSNPSEDQ VDPRLIDGK UniProtKB: Insulin receptor |
-Macromolecule #2: Insulin
| Macromolecule | Name: Insulin / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.383698 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GIVEQCCTSI CSLYQLENYC N UniProtKB: Insulin |
-Macromolecule #3: Insulin
| Macromolecule | Name: Insulin / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.433953 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: FVNQHLCGSH LVEALYLVCG ERGFFYTPKT UniProtKB: Insulin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 Component:
| ||||||||||||
| Grid | Model: C-flat-2/2 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 297 K / Instrument: LEICA EM GP / Details: Blotted for 3s prior to plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average exposure time: 15.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7pg0: |
 Movie
Movie Controller
Controller


































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)