[English] 日本語
 Yorodumi
Yorodumi- EMDB-13233: Cryo-Em structure of the hexameric RUVBL1-RUVBL2 in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13233 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-Em structure of the hexameric RUVBL1-RUVBL2 in complex with ZNHIT2 | ||||||||||||
 Map data Map data | RUVBL1-RUVBL2 AAA ring map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | U5 assembly / protein complex assembly / R2TP / cochaperone / Spliceosome / SPLICING | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpromoter-enhancer loop anchoring activity / telomerase RNA localization to Cajal body / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / Swr1 complex / Ino80 complex ...promoter-enhancer loop anchoring activity / telomerase RNA localization to Cajal body / regulation of DNA strand elongation / positive regulation of telomere maintenance in response to DNA damage / establishment of protein localization to chromatin / R2TP complex / dynein axonemal particle / RPAP3/R2TP/prefoldin-like complex / Swr1 complex / Ino80 complex / regulation of double-strand break repair / box C/D snoRNP assembly / regulation of chromosome organization / NuA4 histone acetyltransferase complex / TFIID-class transcription factor complex binding / regulation of DNA replication / MLL1 complex / regulation of embryonic development / Telomere Extension By Telomerase / protein folding chaperone complex / RNA polymerase II core promoter sequence-specific DNA binding / regulation of DNA repair / positive regulation of double-strand break repair via homologous recombination / Deposition of new CENPA-containing nucleosomes at the centromere / DNA helicase activity / TBP-class protein binding / telomere maintenance / positive regulation of DNA repair / cellular response to estradiol stimulus / euchromatin / negative regulation of canonical Wnt signaling pathway / Formation of the beta-catenin:TCF transactivating complex / ADP binding / beta-catenin binding / chromatin DNA binding / DNA Damage Recognition in GG-NER / nuclear matrix / cellular response to UV / transcription corepressor activity / UCH proteinases / unfolded protein binding / positive regulation of canonical Wnt signaling pathway / nucleosome / protein folding / HATs acetylate histones / ATPase binding / regulation of apoptotic process / DNA recombination / spermatogenesis / DNA helicase / transcription coactivator activity / regulation of cell cycle / Ub-specific processing proteases / protein stabilization / nuclear speck / ciliary basal body / cadherin binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / chromatin remodeling / ribonucleoprotein complex / cell division / DNA repair / centrosome / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / positive regulation of DNA-templated transcription / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / extracellular exosome / nucleoplasm / ATP binding / identical protein binding / membrane / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | ||||||||||||
 Authors Authors | Llorca O / Serna M | ||||||||||||
| Funding support |  Spain, 3 items Spain, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: CryoEM of RUVBL1-RUVBL2-ZNHIT2, a complex that interacts with pre-mRNA-processing-splicing factor 8. Authors: Marina Serna / Ana González-Corpas / Sofía Cabezudo / Andrés López-Perrote / Gianluca Degliesposti / Eduardo Zarzuela / J Mark Skehel / Javier Muñoz / Oscar Llorca /   Abstract: Biogenesis of the U5 small nuclear ribonucleoprotein (snRNP) is an essential and highly regulated process. In particular, PRPF8, one of U5 snRNP main components, requires HSP90 working in concert ...Biogenesis of the U5 small nuclear ribonucleoprotein (snRNP) is an essential and highly regulated process. In particular, PRPF8, one of U5 snRNP main components, requires HSP90 working in concert with R2TP, a cochaperone complex containing RUVBL1 and RUVBL2 AAA-ATPases, and additional factors that are still poorly characterized. Here, we use biochemistry, interaction mapping, mass spectrometry and cryoEM to study the role of ZNHIT2 in the regulation of the R2TP chaperone during the biogenesis of PRPF8. ZNHIT2 forms a complex with R2TP which depends exclusively on the direct interaction of ZNHIT2 with the RUVBL1-RUVBL2 ATPases. The cryoEM analysis of this complex reveals that ZNHIT2 alters the conformation and nucleotide state of RUVBL1-RUVBL2, affecting its ATPase activity. We characterized the interactions between R2TP, PRPF8, ZNHIT2, ECD and AAR2 proteins. Interestingly, PRPF8 makes a direct interaction with R2TP and this complex can incorporate ZNHIT2 and other proteins involved in the biogenesis of PRPF8 such as ECD and AAR2. Together, these results show that ZNHIT2 participates in the assembly of the U5 snRNP as part of a network of contacts between assembly factors required for PRPF8 biogenesis and the R2TP-HSP90 chaperone, while concomitantly regulating the structure and nucleotide state of R2TP. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13233.map.gz emd_13233.map.gz | 89.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13233-v30.xml emd-13233-v30.xml emd-13233.xml emd-13233.xml | 26.4 KB 26.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_13233.png emd_13233.png | 67.5 KB | ||
| Filedesc metadata |  emd-13233.cif.gz emd-13233.cif.gz | 6 KB | ||
| Others |  emd_13233_additional_1.map.gz emd_13233_additional_1.map.gz emd_13233_additional_2.map.gz emd_13233_additional_2.map.gz emd_13233_additional_3.map.gz emd_13233_additional_3.map.gz emd_13233_additional_4.map.gz emd_13233_additional_4.map.gz emd_13233_additional_5.map.gz emd_13233_additional_5.map.gz emd_13233_half_map_1.map.gz emd_13233_half_map_1.map.gz emd_13233_half_map_2.map.gz emd_13233_half_map_2.map.gz | 167.9 MB 140.5 MB 140.7 MB 140.8 MB 166.3 MB 164.9 MB 164.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13233 http://ftp.pdbj.org/pub/emdb/structures/EMD-13233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13233 | HTTPS FTP |
-Related structure data
| Related structure data |  7p6xMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13233.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13233.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
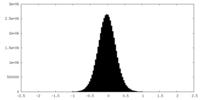
| Annotation | RUVBL1-RUVBL2 AAA ring map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
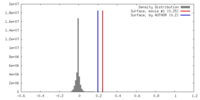
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: RUVBL1-RUVBL2 AAA ring sharpened map
| File | emd_13233_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
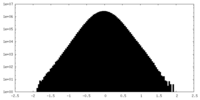
| Annotation | RUVBL1-RUVBL2 AAA ring sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
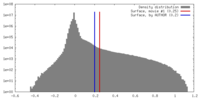
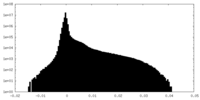
| Density Histograms |
-Additional map: RUVBL1-RUVBL2-ZNHIT2 consensus map
| File | emd_13233_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
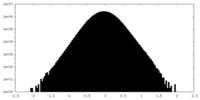
| Annotation | RUVBL1-RUVBL2-ZNHIT2 consensus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
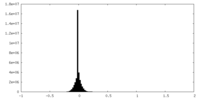
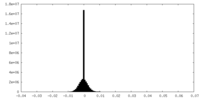
| Density Histograms |
-Additional map: RUVBL-1RUVBL2-ZNHIT2 consensus half map 1
| File | emd_13233_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL-1RUVBL2-ZNHIT2 consensus half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
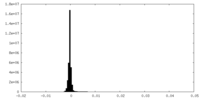
| Density Histograms |
-Additional map: RUVBL-1RUVBL2-ZNHIT2 consensus half map 2
| File | emd_13233_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL-1RUVBL2-ZNHIT2 consensus half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_13233_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
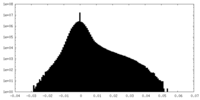
| Density Histograms |
-Half map: RUVBL1-RUVBL2 AAA ring half map 1
| File | emd_13233_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
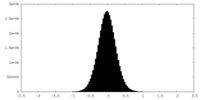
| Density Histograms |
-Half map: RUVBL1-RUVBL2 AAA ring half map 2
| File | emd_13233_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RUVBL1-RUVBL2 AAA ring half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2
| Entire | Name: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2 |
|---|---|
| Components |
|
-Supramolecule #1: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2
| Supramolecule | Name: Hexameric RUVBL1-RUVBL2 AAA ring in complex with ZNHIT2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: RuvB-like 1
| Macromolecule | Name: RuvB-like 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.296914 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK ...String: MKIEEVKSTT KTQRIASHSH VKGLGLDESG LAKQAASGLV GQENAREACG VIVELIKSKK MAGRAVLLAG PPGTGKTALA LAIAQELGS KVPFCPMVGS EVYSTEIKKT EVLMENFRRA IGLRIKETKE VYEGEVTELT PCETENPMGG YGKTISHVII G LKTAKGTK QLKLDPSIFE SLQKERVEAG DVIYIEANSG AVKRQGRCDT YATEFDLEAE EYVPLPKGDV HKKKEIIQDV TL HDLDVAN ARPQGGQDIL SMMGQLMKPK KTEITDKLRG EINKVVNKYI DQGIAELVPG VLFVDEVHML DIECFTYLHR ALE SSIAPI VIFASNRGNC VIRGTEDITS PHGIPLDLLD RVMIIRTMLY TPQEMKQIIK IRAQTEGINI SEEALNHLGE IGTK TTLRY SVQLLTPANL LAKINGKDSI EKEHVEEISE LFYDAKSSAK ILADQQDKYM K UniProtKB: RuvB-like 1 |
-Macromolecule #2: RuvB-like 2
| Macromolecule | Name: RuvB-like 2 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.222465 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ...String: MATVTATTKV PEIRDVTRIE RIGAHSHIRG LGLDDALEPR QASQGMVGQL AARRAAGVVL EMIREGKIAG RAVLIAGQPG TGKTAIAMG MAQALGPDTP FTAIAGSEIF SLEMSKTEAL TQAFRRSIGV RIKEETEIIE GEVVEIQIDR PATGTGSKVG K LTLKTTEM ETIYDLGTKM IESLTKDKVQ AGDVITIDKA TGKISKLGRS FTRARDYDAM GSQTKFVQCP DGELQKRKEV VH TVSLHEI DVINSRTQGF LALFSGDTGE IKSEVREQIN AKVAEWREEG KAEIIPGVLF IDEVHMLDIE SFSFLNRALE SDM APVLIM ATNRGITRIR GTSYQSPHGI PIDLLDRLLI VSTTPYSEKD TKQILRIRCE EEDVEMSEDA YTVLTRIGLE TSLR YAIQL ITAASLVCRK RKGTEVQVDD IKRVYSLFLD ESRSTQYMKE YQDAFLFNEL KGETMDTS UniProtKB: RuvB-like 2 |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 3 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE / Details: Ab initio (Cryosparc) |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 173905 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)