+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13154 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 2.29 A Mycobacterium tuberculosis EspB. | |||||||||||||||
 Map data Map data | Local B-factor sharpened map by Locspiral. | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Cryo-EM / EspB / ESX-1 / Preferential orientation / PROTEIN TRANSPORT | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein secretion by the type VII secretion system / symbiont-mediated suppression of host autophagy / biological process involved in interaction with host / extracellular region / identical protein binding Similarity search - Function | |||||||||||||||
| Biological species |  Mycobacterium tuberculosis H37RV (bacteria) Mycobacterium tuberculosis H37RV (bacteria) | |||||||||||||||
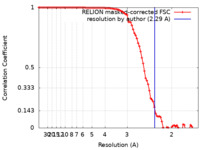
| Method | single particle reconstruction / cryo EM / Resolution: 2.29 Å | |||||||||||||||
 Authors Authors | Gijsbers A / Zhang Y | |||||||||||||||
| Funding support |  Netherlands, European Union, Netherlands, European Union,  Mexico, 4 items Mexico, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Curr Res Struct Biol / Year: 2021 Journal: Curr Res Struct Biol / Year: 2021Title: Priming mycobacterial ESX-secreted protein B to form a channel-like structure. Authors: Abril Gijsbers / Vanesa Vinciauskaite / Axel Siroy / Ye Gao / Giancarlo Tria / Anjusha Mathew / Nuria Sánchez-Puig / Carmen López-Iglesias / Peter J Peters / Raimond B G Ravelli /   Abstract: ESX-1 is a major virulence factor of , a secretion machinery directly involved in the survival of the microorganism from the immune system defence. It disrupts the phagosome membrane of the host cell ...ESX-1 is a major virulence factor of , a secretion machinery directly involved in the survival of the microorganism from the immune system defence. It disrupts the phagosome membrane of the host cell through a contact-dependent mechanism. Recently, the structure of the inner-membrane core complex of the homologous ESX-3 and ESX-5 was resolved; however, the elements involved in the secretion through the outer membrane or those acting on the host cell membrane are unknown. Protein substrates might form this missing element. Here, we describe the oligomerisation process of the ESX-1 substrate EspB, which occurs upon cleavage of its C-terminal region and is favoured by an acidic environment. Cryo-electron microscopy data shows that quaternary structure of EspB is conserved across slow growing species, but not in the fast growing . EspB assembles into a channel with dimensions and characteristics suitable for the transit of ESX-1 substrates, as shown by the presence of another EspB trapped within. Our results provide insight into the structure and assembly of EspB, and suggests a possible function as a structural element of ESX-1. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13154.map.gz emd_13154.map.gz | 11.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13154-v30.xml emd-13154-v30.xml emd-13154.xml emd-13154.xml | 21.5 KB 21.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13154_fsc.xml emd_13154_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13154.png emd_13154.png | 212.5 KB | ||
| Masks |  emd_13154_msk_1.map emd_13154_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13154.cif.gz emd-13154.cif.gz | 6.4 KB | ||
| Others |  emd_13154_additional_1.map.gz emd_13154_additional_1.map.gz emd_13154_half_map_1.map.gz emd_13154_half_map_1.map.gz emd_13154_half_map_2.map.gz emd_13154_half_map_2.map.gz | 7 MB 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13154 http://ftp.pdbj.org/pub/emdb/structures/EMD-13154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13154 | HTTPS FTP |
-Related structure data
| Related structure data |  7p13MC  7p0zC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13154.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13154.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local B-factor sharpened map by Locspiral. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
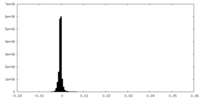
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13154_msk_1.map emd_13154_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
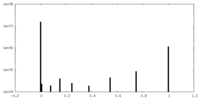
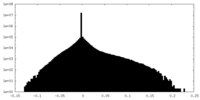
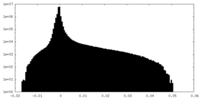
| Density Histograms |
-Additional map: Global B-factor sharpened map from Relion postprocessing.
| File | emd_13154_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Global B-factor sharpened map from Relion postprocessing. | ||||||||||||
| Projections & Slices |
| ||||||||||||
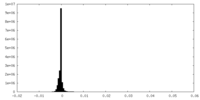
| Density Histograms |
-Half map: Unmasked halfmap 1.
| File | emd_13154_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unmasked halfmap 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
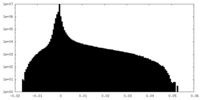
| Density Histograms |
-Half map: Unmasked halfmap 2.
| File | emd_13154_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unmasked halfmap 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : heptamer of EspB
| Entire | Name: heptamer of EspB |
|---|---|
| Components |
|
-Supramolecule #1: heptamer of EspB
| Supramolecule | Name: heptamer of EspB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37RV (bacteria) Mycobacterium tuberculosis H37RV (bacteria) |
| Molecular weight | Theoretical: 220 KDa |
-Macromolecule #1: ESX-1 secretion-associated protein EspB
| Macromolecule | Name: ESX-1 secretion-associated protein EspB / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37RV (bacteria) Mycobacterium tuberculosis H37RV (bacteria) |
| Molecular weight | Theoretical: 31.561842 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SMHTQSQTVT VDQQEILNRA NEVEAPMADP PTDVPITPCE LTAAKNAAQQ LVLSADNMRE YLAAGAKERQ RLATSLRNAA KAYGEVDEE AATALDNDGE GTVQAESAGA VGGDSSAELT DTPRVATAGE PNFMDLKEAA RKLETGDQGA SLAHFADGWN T FNLTLQGD ...String: SMHTQSQTVT VDQQEILNRA NEVEAPMADP PTDVPITPCE LTAAKNAAQQ LVLSADNMRE YLAAGAKERQ RLATSLRNAA KAYGEVDEE AATALDNDGE GTVQAESAGA VGGDSSAELT DTPRVATAGE PNFMDLKEAA RKLETGDQGA SLAHFADGWN T FNLTLQGD VKRFRGFDNW EGDAATACEA SLDQQRQWIL HMAKLSAAMA KQAQYVAQLH VWARREHPTY EDIVGLERLY AE NPSARDQ ILPVYAEYQQ RSEKVLTEYN NKAALEPVNP PKPPPAIKID PP UniProtKB: ESX-1 secretion-associated protein EspB |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 5.5 Component:
| |||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Support film - Film thickness: 2000 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.01 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Temperature | Min: 90.0 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Details | Basic direct alignments were done as well as astigmatism and coma alignment using AutoCTF |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 2334 / Average exposure time: 1.8 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)