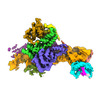
登録情報 データベース : EMDB / ID : EMD-12799タイトル SRP-SR at the distal site conformation SRP-SR complex from 3D focused refinement on ribosome signal subtracted images 複合体 : SRP-SR complex複合体 : SRP-SR complexRNA : x 1種タンパク質・ペプチド : x 4種複合体 : EM14S01-3B_G0054400.mRNA.1.CDS.1複合体 : Signal recognition particle receptor subunitリガンド : x 3種 / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Canis lupus familiaris (イヌ) / Saccharomyces cerevisiae (パン酵母) / Oryctolagus cuniculus (ウサギ)手法 / / 解像度 : 3.9 Å Jomaa A / Ban N 資金援助 Organization Grant number 国 Swiss National Science Foundation
ジャーナル : Cell Rep / 年 : 2021タイトル : Molecular mechanism of cargo recognition and handover by the mammalian signal recognition particle.
著者 :
Ahmad Jomaa / Simon Eitzinger / Zikun Zhu / Sowmya Chandrasekar / Kan Kobayashi / Shu-Ou Shan / Nenad Ban / 要旨 :
Co-translational protein targeting to membranes by the signal recognition particle (SRP) is a universally conserved pathway from bacteria to humans. In mammals, SRP and its receptor (SR) have many ... Co-translational protein targeting to membranes by the signal recognition particle (SRP) is a universally conserved pathway from bacteria to humans. In mammals, SRP and its receptor (SR) have many additional RNA features and protein components compared to the bacterial system, which were recently shown to play regulatory roles. Due to its complexity, the mammalian SRP targeting process is mechanistically not well understood. In particular, it is not clear how SRP recognizes translating ribosomes with exposed signal sequences and how the GTPase activity of SRP and SR is regulated. Here, we present electron cryo-microscopy structures of SRP and SRP·SR in complex with the translating ribosome. The structures reveal the specific molecular interactions between SRP and the emerging signal sequence and the elements that regulate GTPase activity of SRP·SR. Our results suggest the molecular mechanism of how eukaryote-specific elements regulate the early and late stages of SRP-dependent protein targeting. 履歴 登録 2021年4月23日 - ヘッダ(付随情報) 公開 2021年7月21日 - マップ公開 2021年7月21日 - 更新 2024年7月10日 - 現状 2024年7月10日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報


 データ登録者
データ登録者 スイス, 1件
スイス, 1件  引用
引用 ジャーナル: Cell Rep / 年: 2021
ジャーナル: Cell Rep / 年: 2021

 ジャーナル: Cell Rep / 年: 2021
ジャーナル: Cell Rep / 年: 2021 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_12799.map.gz
emd_12799.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-12799-v30.xml
emd-12799-v30.xml emd-12799.xml
emd-12799.xml EMDBヘッダ
EMDBヘッダ emd_12799.png
emd_12799.png emd-12799.cif.gz
emd-12799.cif.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-12799
http://ftp.pdbj.org/pub/emdb/structures/EMD-12799 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12799
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12799 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_12799.map.gz / 形式: CCP4 / 大きさ: 343 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_12799.map.gz / 形式: CCP4 / 大きさ: 343 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析 ムービー
ムービー コントローラー
コントローラー


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)

























