[English] 日本語
 Yorodumi
Yorodumi- EMDB-12366: Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12366 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA2-Fab (phosphatidylcholine-bound, occluded conformation) | ||||||||||||
 Map data Map data | Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA2-Fab (phosphatidylcholine-bound, occluded conformation) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ABCB4 / MDR3 / nanodisc / lipid transporter / transporter / phosphatidylcholine / PROTEIN TRANSPORT | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to fenofibrate / Defective ABCB4 causes PFIC3, ICP3 and GBD1 / positive regulation of phospholipid transport / positive regulation of phospholipid translocation / bile acid secretion / phospholipid transporter activity / cellular response to bile acid / phosphatidylcholine floppase activity / intercellular canaliculus / P-type phospholipid transporter ...response to fenofibrate / Defective ABCB4 causes PFIC3, ICP3 and GBD1 / positive regulation of phospholipid transport / positive regulation of phospholipid translocation / bile acid secretion / phospholipid transporter activity / cellular response to bile acid / phosphatidylcholine floppase activity / intercellular canaliculus / P-type phospholipid transporter / clathrin-coated vesicle / positive regulation of cholesterol transport / phospholipid translocation / lipid homeostasis / ATPase-coupled transmembrane transporter activity / ABC-type transporter activity / lipid metabolic process / PPARA activates gene expression / ABC-family proteins mediated transport / transmembrane transport / apical plasma membrane / membrane raft / focal adhesion / ATP hydrolysis activity / extracellular exosome / nucleoplasm / ATP binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
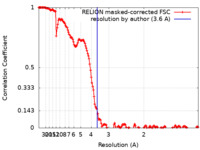
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Nosol K / Locher KP | ||||||||||||
| Funding support |  Switzerland, Switzerland,  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Structures of ABCB4 provide insight into phosphatidylcholine translocation. Authors: Kamil Nosol / Rose Bang-Sørensen / Rossitza N Irobalieva / Satchal K Erramilli / Bruno Stieger / Anthony A Kossiakoff / Kaspar P Locher /   Abstract: ABCB4 is expressed in hepatocytes and translocates phosphatidylcholine into bile canaliculi. The mechanism of specific lipid recruitment from the canalicular membrane, which is essential to mitigate ...ABCB4 is expressed in hepatocytes and translocates phosphatidylcholine into bile canaliculi. The mechanism of specific lipid recruitment from the canalicular membrane, which is essential to mitigate the cytotoxicity of bile salts, is poorly understood. We present cryogenic electron microscopy structures of human ABCB4 in three distinct functional conformations. An apo-inward structure reveals how phospholipid can be recruited from the inner leaflet of the membrane without flipping its orientation. An occluded structure reveals a single phospholipid molecule in a central cavity. Its choline moiety is stabilized by cation-π interactions with an essential tryptophan residue, rationalizing the specificity of ABCB4 for phosphatidylcholine. In an inhibitor-bound structure, a posaconazole molecule blocks phospholipids from reaching the central cavity. Using a proteoliposome-based translocation assay with fluorescently labeled phosphatidylcholine analogs, we recapitulated the substrate specificity of ABCB4 in vitro and confirmed the role of the key tryptophan residue. Our results provide a structural basis for understanding an essential translocation step in the generation of bile and its sensitivity to azole drugs. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12366.map.gz emd_12366.map.gz | 310.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12366-v30.xml emd-12366-v30.xml emd-12366.xml emd-12366.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12366_fsc.xml emd_12366_fsc.xml | 16 KB | Display |  FSC data file FSC data file |
| Images |  emd_12366.png emd_12366.png | 117.5 KB | ||
| Filedesc metadata |  emd-12366.cif.gz emd-12366.cif.gz | 7.3 KB | ||
| Others |  emd_12366_additional_1.map.gz emd_12366_additional_1.map.gz | 308.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12366 http://ftp.pdbj.org/pub/emdb/structures/EMD-12366 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12366 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12366 | HTTPS FTP |
-Related structure data
| Related structure data |  7nivMC  7niuC  7niwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12366.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12366.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA2-Fab (phosphatidylcholine-bound, occluded conformation) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
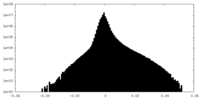
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.66 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
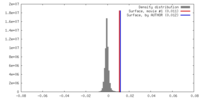
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab...
| File | emd_12366_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA2-Fab (phosphatidylcholine-bound, occluded conformation) | ||||||||||||
| Projections & Slices |
| ||||||||||||
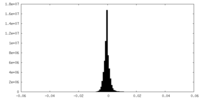
| Density Histograms |
- Sample components
Sample components
+Entire : Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA...
+Supramolecule #1: Nanodisc reconstituted human ABCB4 in complex with 4B1-Fab and QA...
+Supramolecule #2: phosphatidylcholine translocator ABCB4
+Supramolecule #3: QA2 Fab-fragment: light and heavy chain
+Supramolecule #4: 4B1 Fab-fragment: light and heavy chain
+Macromolecule #1: QA2 Fab-fragment light chain
+Macromolecule #2: QA2 Fab-fragment light chain
+Macromolecule #3: Isoform 2 of Phosphatidylcholine translocator ABCB4
+Macromolecule #4: 4B1 Fab-fragment light chain
+Macromolecule #5: 4B1 Fab-fragment light chain
+Macromolecule #6: 1,2-DILINOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #7: CHOLESTEROL
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.6 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 80.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)