[English] 日本語
 Yorodumi
Yorodumi- EMDB-12294: CryoEM structure of disease related M854K MDA5-dsRNA filament in ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12294 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of disease related M854K MDA5-dsRNA filament in complex with ADP-AlF4(minor class) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMDA-5 signaling pathway / positive regulation of response to cytokine stimulus / Ub-specific processing proteases / negative regulation of viral genome replication / type I interferon-mediated signaling pathway / cellular response to exogenous dsRNA / pattern recognition receptor activity / protein complex oligomerization / positive regulation of interferon-alpha production / protein sumoylation ...MDA-5 signaling pathway / positive regulation of response to cytokine stimulus / Ub-specific processing proteases / negative regulation of viral genome replication / type I interferon-mediated signaling pathway / cellular response to exogenous dsRNA / pattern recognition receptor activity / protein complex oligomerization / positive regulation of interferon-alpha production / protein sumoylation / ribonucleoprotein complex binding / antiviral innate immune response / positive regulation of interferon-beta production / cellular response to virus / positive regulation of interleukin-6 production / response to virus / positive regulation of tumor necrosis factor production / double-stranded RNA binding / defense response to virus / RNA helicase activity / single-stranded RNA binding / RNA helicase / protein domain specific binding / innate immune response / ATP hydrolysis activity / mitochondrion / DNA binding / zinc ion binding / ATP binding / identical protein binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Pseudomonas virus phi6 Pseudomonas virus phi6 | |||||||||
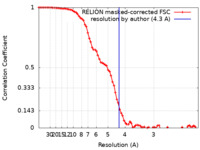
| Method | helical reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Yu Q / Modis Y | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: MDA5 disease variant M854K prevents ATP-dependent structural discrimination of viral and cellular RNA. Authors: Qin Yu / Alba Herrero Del Valle / Rahul Singh / Yorgo Modis /   Abstract: Our innate immune responses to viral RNA are vital defenses. Long cytosolic double-stranded RNA (dsRNA) is recognized by MDA5. The ATPase activity of MDA5 contributes to its dsRNA binding selectivity. ...Our innate immune responses to viral RNA are vital defenses. Long cytosolic double-stranded RNA (dsRNA) is recognized by MDA5. The ATPase activity of MDA5 contributes to its dsRNA binding selectivity. Mutations that reduce RNA selectivity can cause autoinflammatory disease. Here, we show how the disease-associated MDA5 variant M854K perturbs MDA5-dsRNA recognition. M854K MDA5 constitutively activates interferon signaling in the absence of exogenous RNA. M854K MDA5 lacks ATPase activity and binds more stably to synthetic Alu:Alu dsRNA. CryoEM structures of MDA5-dsRNA filaments at different stages of ATP hydrolysis show that the K854 sidechain forms polar bonds that constrain the conformation of MDA5 subdomains, disrupting key steps in the ATPase cycle- RNA footprint expansion and helical twist modulation. The M854K mutation inhibits ATP-dependent RNA proofreading via an allosteric mechanism, allowing MDA5 to form signaling complexes on endogenous RNAs. This work provides insights on how MDA5 recognizes dsRNA in health and disease. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12294.map.gz emd_12294.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12294-v30.xml emd-12294-v30.xml emd-12294.xml emd-12294.xml | 21.3 KB 21.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12294_fsc.xml emd_12294_fsc.xml | 7.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_12294.png emd_12294.png | 155.4 KB | ||
| Masks |  emd_12294_msk_1.map emd_12294_msk_1.map | 40.6 MB |  Mask map Mask map | |
| Others |  emd_12294_half_map_1.map.gz emd_12294_half_map_1.map.gz emd_12294_half_map_2.map.gz emd_12294_half_map_2.map.gz | 31 MB 31 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12294 http://ftp.pdbj.org/pub/emdb/structures/EMD-12294 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12294 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12294 | HTTPS FTP |
-Related structure data
| Related structure data |  7nicMC  7bkpC  7bkqC  7ngaC  7niqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10664 (Title: Disease variant M854K MDA5-dsRNA filaments in complex with ADP-AlF4 EMPIAR-10664 (Title: Disease variant M854K MDA5-dsRNA filaments in complex with ADP-AlF4Data size: 2.5 TB Data #1: Unaligned multi-frame micrographs of MDA5 variant M854K bound to dsRNA and ADP-ALF4 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12294.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12294.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12294_msk_1.map emd_12294_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_12294_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_12294_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : M854K MDA5-dsRNA filament in complex with ADP-AlF4(major class)
| Entire | Name: M854K MDA5-dsRNA filament in complex with ADP-AlF4(major class) |
|---|---|
| Components |
|
-Supramolecule #1: M854K MDA5-dsRNA filament in complex with ADP-AlF4(major class)
| Supramolecule | Name: M854K MDA5-dsRNA filament in complex with ADP-AlF4(major class) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Experimental: 2 kDa/nm |
-Supramolecule #2: INTERFERON-INDUCED HELICASE C DOMAIN-CONTAINING PROTEIN
| Supramolecule | Name: INTERFERON-INDUCED HELICASE C DOMAIN-CONTAINING PROTEIN type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Supramolecule #3: RNA
| Supramolecule | Name: RNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #4: RNA
| Supramolecule | Name: RNA / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: INTERFERON-INDUCED HELICASE C DOMAIN-CONTAINING PROTEIN
| Macromolecule | Name: INTERFERON-INDUCED HELICASE C DOMAIN-CONTAINING PROTEIN type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: RNA helicase |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: LQLRPYQMEV AQPALDGKNI IICLPTGSGK TRVAVYITKD HLDKKKQASE SGKVIVLVNK VMLAEQLFRK EFNPYLKKWY RIIGLSGDTQ LKISFPEVVK SYDVIISTAQ ILENSLLNLE SGDDDGVQLS DFSLIIIDEC HHTNKEAVYN NIMRRYLKQK LRNNDLKKQN ...String: LQLRPYQMEV AQPALDGKNI IICLPTGSGK TRVAVYITKD HLDKKKQASE SGKVIVLVNK VMLAEQLFRK EFNPYLKKWY RIIGLSGDTQ LKISFPEVVK SYDVIISTAQ ILENSLLNLE SGDDDGVQLS DFSLIIIDEC HHTNKEAVYN NIMRRYLKQK LRNNDLKKQN KPAIPLPQIL GLTASPGVGA AKKQSEAEKH ILNICANLDA FTIKTVKENL GQLKHQIKEP CKKFVIADDT RENPFKEKLL EIMASIQTYC QKSPMSDFGT QHYEQWAIQM EKKAAKDGNR KDRVCAEHLR KYNEALQIND TIRMIDAYSH LETFYTDEKE KKFAVLNDSK KSLKLDETDE FLMNLFFDNK KMLKKLAENP KYENEKLIKL RNTILEQFTR SEESSRGIIF TKTRQSTYAL SQWIMENAKF AEVGVKAHHL IGAGHSSEVK PMTQTEQKEV ISKFRTGEIN LLIATTVAEE GLDIKECNIV IRYGLVTNEI AMVQARGRAR ADESTYVLVT SSGSGVTERE IVNDFREKMK YKAINRVQNM KPEEYAHKIL ELQVQSILEK KMKVKRSIAK QYNDNPSLIT LLCKNCSMLV CSGENIHVIE KMHHVNMTPE FKGLYIVREN KALQKKFADY QTNGEIICKC GQAWGTMMVH KGLDLPCLKI RNFVVNFKNN SPKKQYKKWV ELPIRFPDLD YSEYCL |
-Macromolecule #2: RNA
| Macromolecule | Name: RNA / type: rna / ID: 2 |
|---|---|
| Source (natural) | Organism:  Pseudomonas virus phi6 Pseudomonas virus phi6 |
| Sequence | String: GUCAAGCCGA GGAGA |
-Macromolecule #3: RNA
| Macromolecule | Name: RNA / type: rna / ID: 3 |
|---|---|
| Source (natural) | Organism:  Pseudomonas virus phi6 Pseudomonas virus phi6 |
| Sequence | String: UCUCCUCGGC UUGAC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.7 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Details: machine step 6 | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.2 K / Instrument: FEI VITROBOT MARK IV Details: Grids were blotted for 3 s; blot force 10, 5, 0, -5, -10. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: OTHER |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 2177 / Average exposure time: 60.0 sec. / Average electron dose: 29.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -3.0 µm / Nominal defocus min: -1.8 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)