[English] 日本語
 Yorodumi
Yorodumi- EMDB-12233: C. thermophilum core structure of mixed 2-oxoglutarate dehydrogen... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12233 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | C. thermophilum core structure of mixed 2-oxoglutarate dehydrogenase complex and branched-chain 2-oxo acid dehydrogenase complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) / Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) /  Chaetomium thermophilum (fungus) Chaetomium thermophilum (fungus) | |||||||||
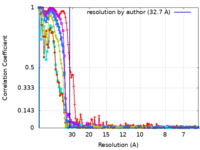
| Method | single particle reconstruction / negative staining / Resolution: 32.7 Å | |||||||||
 Authors Authors | Kyrilis FL / Semchonok DA / Skalidis I / Tueting C / Hamdi F / O'Reilly FJ / Rappsilber J / Kastritis PL | |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Integrative structure of a 10-megadalton eukaryotic pyruvate dehydrogenase complex from native cell extracts. Authors: Fotis L Kyrilis / Dmitry A Semchonok / Ioannis Skalidis / Christian Tüting / Farzad Hamdi / Francis J O'Reilly / Juri Rappsilber / Panagiotis L Kastritis /   Abstract: The pyruvate dehydrogenase complex (PDHc) is a giant enzymatic assembly involved in pyruvate oxidation. PDHc components have been characterized in isolation, but the complex's quaternary structure ...The pyruvate dehydrogenase complex (PDHc) is a giant enzymatic assembly involved in pyruvate oxidation. PDHc components have been characterized in isolation, but the complex's quaternary structure has remained elusive due to sheer size, heterogeneity, and plasticity. Here, we identify fully assembled Chaetomium thermophilum α-keto acid dehydrogenase complexes in native cell extracts and characterize their domain arrangements utilizing mass spectrometry, activity assays, crosslinking, electron microscopy (EM), and computational modeling. We report the cryo-EM structure of the PDHc core and observe unique features of the previously unknown native state. The asymmetric reconstruction of the 10-MDa PDHc resolves spatial proximity of its components, agrees with stoichiometric data (60 E2p:12 E3BP:∼20 E1p: ≤ 12 E3), and proposes a minimum reaction path among component enzymes. The PDHc shows the presence of a dynamic pyruvate oxidation compartment, organized by core and peripheral protein species. Our data provide a framework for further understanding PDHc and α-keto acid dehydrogenase complex structure and function. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12233.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12233.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Additional map: #7
+Additional map: #3
+Additional map: #6
+Additional map: #21
+Additional map: #20
+Additional map: #19
+Additional map: #18
+Additional map: #17
+Additional map: #16
+Additional map: #15
+Additional map: #14
+Additional map: #13
+Additional map: #4
+Additional map: #5
+Additional map: #12
+Additional map: #11
+Additional map: #10
+Additional map: #9
+Additional map: #8
+Additional map: #2
+Additional map: #1
+Half map: #2
+Half map: #1
- Sample components
Sample components
-Entire : Native core of the mixed oxoglutarate/branched-chain keto-acid de...
| Entire | Name: Native core of the mixed oxoglutarate/branched-chain keto-acid dehydrogenase complex core from C. thermophilum |
|---|---|
| Components |
|
-Supramolecule #1: Native core of the mixed oxoglutarate/branched-chain keto-acid de...
| Supramolecule | Name: Native core of the mixed oxoglutarate/branched-chain keto-acid dehydrogenase complex core from C. thermophilum type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
| Molecular weight | Theoretical: 2 MDa |
-Macromolecule #1: succinyl-CoA:enzyme-N6-(dihydrolipoyl)lysine S-succinyltransferase
| Macromolecule | Name: succinyl-CoA:enzyme-N6-(dihydrolipoyl)lysine S-succinyltransferase type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue succinyltransferase |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) / Strain: DSM 1495 Chaetomium thermophilum (fungus) / Strain: DSM 1495 |
| Sequence | String: MLYRGLRMAA RAAPKFAVLN THAALRQLPL QFQHVRTYAD KIIKVPQMAE SITEGTLKQW NKAVGDYVEA DEEIATIETD KIDVAVNAPE AGVIKEFFVN EEDTVLVGQD LVRLEVGGEK PAEAAKEQPK AAAPEPKVEE KKVPEAPAPE PSKTAAPAPA PPKQEAPASP ...String: MLYRGLRMAA RAAPKFAVLN THAALRQLPL QFQHVRTYAD KIIKVPQMAE SITEGTLKQW NKAVGDYVEA DEEIATIETD KIDVAVNAPE AGVIKEFFVN EEDTVLVGQD LVRLEVGGEK PAEAAKEQPK AAAPEPKVEE KKVPEAPAPE PSKTAAPAPA PPKQEAPASP KPASKPAETP AVTLGNREER RVKMNRMRLR IAERLKQSQN TAASLTTFNE VDMSALIEFR NKYKDEVLKK TGVKLGFMSA FSRAVVLAIR DLPVVNASIE GPNGGDTIVY RDYVDISVAV ATEKGLVTPV VRNAETMDLI TIEKTIAELG KKARDGKLTI EDMAGGTFTI SNGGVFGSLM GTPIINLPQS AVLGLHAIKE RPVAVNGKVE IRPMMYLALT YDHRLLDGRE AVQFLVKVKE YIEDPRKMLL |
-Macromolecule #2: 2-methylpropanoyl-CoA:enzyme-N6-(dihydrolipoyl)lysine S-(2-methyl...
| Macromolecule | Name: 2-methylpropanoyl-CoA:enzyme-N6-(dihydrolipoyl)lysine S-(2-methylpropanoyl)transferase type: protein_or_peptide / ID: 2 / Enantiomer: LEVO EC number: dihydrolipoyllysine-residue (2-methylpropanoyl)transferase |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) / Strain: DSM 1495 Chaetomium thermophilum (fungus) / Strain: DSM 1495 |
| Sequence | String: MRSWFFSRVL RQQWRVVRGL ARRAAAPTST TPLSVLSPLS AQAFHASRTL QVVKPVLLAD IGEGIVECEI IQWFVEPGAR VEEFQPLCEV QSDKASVEIT SRFAGVVKKL YYEAGEMAKV GKPFVDIDIV DGVVKEDSSA TVPIDSTPAL DKTLEAPVRP PAEGTAEAQV ...String: MRSWFFSRVL RQQWRVVRGL ARRAAAPTST TPLSVLSPLS AQAFHASRTL QVVKPVLLAD IGEGIVECEI IQWFVEPGAR VEEFQPLCEV QSDKASVEIT SRFAGVVKKL YYEAGEMAKV GKPFVDIDIV DGVVKEDSSA TVPIDSTPAL DKTLEAPVRP PAEGTAEAQV ATATNEPTSP SKSKGKCATL ATPAVRHLSK QLGVDIAEVD GTGKDGRVLK EDIYRFVERR EAAAKQAPAT QPTASSPTPS SITSPVEGST AGSQQETPVP LTRTQEMMFK TMTRSLSIPH FLYADEVDFT KLVDLRSRLN NVLSKHGIND GQSVKLTYLP FIIKAVSMAL YQYPILNARV EIPENGNGKP MLIHRSQHNI GVAMDTPSGL LVPVIKNVGN LNILGIASEL ARLQSLAMAG KLTPQDMSGG TITVSNIGNI GGTYLSPVIV EREVAILGIG RMRTVPAFST VPGEEDKVVK KQICNFSWSA DHRVIDGATM ARAAEVVRSI VEEPDVMVMH LR |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Concentration: 200.0 mM / Component - Formula: NH4CH2COOH / Component - Name: Ammonium acetate |
| Staining | Type: NEGATIVE / Material: Uranyl Acetate |
| Grid | Model: C-flat / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 10.0 nm / Pretreatment - Type: GLOW DISCHARGE |
| Details | fractionated native cell extract |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Temperature | Min: 77.15 K / Max: 120.0 K |
| Details | Coma Free kept better than 200nm in EPU |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 14.0 µm / Number grids imaged: 1 / Number real images: 1926 / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 34739 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)