[English] 日本語
 Yorodumi
Yorodumi- EMDB-10906: Structure of the P+9 ArfB-ribosome complex in the post-hydrolysis... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10906 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the P+9 ArfB-ribosome complex in the post-hydrolysis state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | translation / ribosome / rescue / release | |||||||||
| Function / homology |  Function and homology information Function and homology informationpeptidyl-tRNA hydrolase / translation release factor activity / peptidyl-tRNA hydrolase activity / stringent response / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation ...peptidyl-tRNA hydrolase / translation release factor activity / peptidyl-tRNA hydrolase activity / stringent response / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / rescue of stalled cytosolic ribosome / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / ribosome assembly / regulation of cell growth / DNA-templated transcription termination / response to radiation / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / defense response to bacterium / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / innate immune response / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / extracellular region / zinc ion binding / metal ion binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
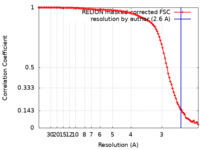
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Chan K-H / Petrychenko V | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Mechanism of ribosome rescue by alternative ribosome-rescue factor B. Authors: Kai-Hsin Chan / Valentyn Petrychenko / Claudia Mueller / Cristina Maracci / Wolf Holtkamp / Daniel N Wilson / Niels Fischer / Marina V Rodnina /  Abstract: Alternative ribosome-rescue factor B (ArfB) rescues ribosomes stalled on non-stop mRNAs by releasing the nascent polypeptide from the peptidyl-tRNA. By rapid kinetics we show that ArfB selects ...Alternative ribosome-rescue factor B (ArfB) rescues ribosomes stalled on non-stop mRNAs by releasing the nascent polypeptide from the peptidyl-tRNA. By rapid kinetics we show that ArfB selects ribosomes stalled on short truncated mRNAs, rather than on longer mRNAs mimicking pausing on rare codon clusters. In combination with cryo-electron microscopy we dissect the multistep rescue pathway of ArfB, which first binds to ribosomes very rapidly regardless of the mRNA length. The selectivity for shorter mRNAs arises from the subsequent slow engagement step, as it requires longer mRNA to shift to enable ArfB binding. Engagement results in specific interactions of the ArfB C-terminal domain with the mRNA entry channel, which activates peptidyl-tRNA hydrolysis by the N-terminal domain. These data reveal how protein dynamics translate into specificity of substrate recognition and provide insights into the action of a putative rescue factor in mitochondria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10906.map.gz emd_10906.map.gz | 461.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10906-v30.xml emd-10906-v30.xml emd-10906.xml emd-10906.xml | 90.4 KB 90.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10906_fsc.xml emd_10906_fsc.xml | 10.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_10906.png emd_10906.png | 144.4 KB | ||
| Masks |  emd_10906_msk_1.map emd_10906_msk_1.map | 91.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-10906.cif.gz emd-10906.cif.gz | 16.2 KB | ||
| Others |  emd_10906_half_map_1.map.gz emd_10906_half_map_1.map.gz emd_10906_half_map_2.map.gz emd_10906_half_map_2.map.gz | 70.6 MB 70.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10906 http://ftp.pdbj.org/pub/emdb/structures/EMD-10906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10906 | HTTPS FTP |
-Related structure data
| Related structure data |  6yssMC  6ysrC  6ystC  6ysuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10443 (Title: Mechanism of Ribosome Rescue by Alternative Release Factor B EMPIAR-10443 (Title: Mechanism of Ribosome Rescue by Alternative Release Factor BData size: 788.2 Data #1: Motion-corrected, dose-weighted micrographs & polished particles of P+9 E. coli ribosome-ArfB complex [micrographs - single frame] Data #2: Motion-corrected, dose-weighted micrographs & extracted particles of P+0 E. coli ribosome-ArfB complex [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10906.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10906.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.6525 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10906_msk_1.map emd_10906_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_10906_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_10906_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Post-hydrolysis state of E. coli ribosome P+9 70S-ArfB-Api137-tRN...
+Supramolecule #1: Post-hydrolysis state of E. coli ribosome P+9 70S-ArfB-Api137-tRN...
+Supramolecule #2: Post-hydrolysis state of E. coli ribosome P+9 70S-ArfB-Api137-tRN...
+Supramolecule #3: Post-hydrolysis state of E. coli ribosome P+9 70S-ArfB-Api137-tRN...
+Supramolecule #4: Post-hydrolysis state of E. coli ribosome P+9 70S-ArfB-Api137-tRN...
+Macromolecule #1: 50S ribosomal protein L32
+Macromolecule #2: 50S ribosomal protein L33
+Macromolecule #3: 50S ribosomal protein L34
+Macromolecule #4: 50S ribosomal protein L35
+Macromolecule #5: 50S ribosomal protein L36
+Macromolecule #6: 50S ribosomal protein L10
+Macromolecule #7: 50S ribosomal protein L31
+Macromolecule #10: 50S ribosomal protein L2
+Macromolecule #11: 50S ribosomal protein L3
+Macromolecule #12: 50S ribosomal protein L4
+Macromolecule #13: 50S ribosomal protein L5
+Macromolecule #14: 50S ribosomal protein L6
+Macromolecule #15: 50S ribosomal protein L9
+Macromolecule #16: 50S ribosomal protein L11
+Macromolecule #17: 50S ribosomal protein L13
+Macromolecule #18: 50S ribosomal protein L14
+Macromolecule #19: 50S ribosomal protein L15
+Macromolecule #20: 50S ribosomal protein L16
+Macromolecule #21: 50S ribosomal protein L17
+Macromolecule #22: 50S ribosomal protein L18
+Macromolecule #23: 50S ribosomal protein L19
+Macromolecule #24: 50S ribosomal protein L20
+Macromolecule #25: 50S ribosomal protein L21
+Macromolecule #26: 50S ribosomal protein L22
+Macromolecule #27: 50S ribosomal protein L23
+Macromolecule #28: 50S ribosomal protein L24
+Macromolecule #29: 50S ribosomal protein L25
+Macromolecule #30: 50S ribosomal protein L27
+Macromolecule #31: 50S ribosomal protein L28
+Macromolecule #32: 50S ribosomal protein L29
+Macromolecule #33: 50S ribosomal protein L30
+Macromolecule #35: 30S ribosomal protein S2
+Macromolecule #36: 30S ribosomal protein S3
+Macromolecule #37: 30S ribosomal protein S4
+Macromolecule #38: 30S ribosomal protein S5
+Macromolecule #39: 30S ribosomal protein S6
+Macromolecule #40: 30S ribosomal protein S7
+Macromolecule #41: 30S ribosomal protein S8
+Macromolecule #42: 30S ribosomal protein S9
+Macromolecule #43: 30S ribosomal protein S10
+Macromolecule #44: 30S ribosomal protein S11
+Macromolecule #45: 30S ribosomal protein S12
+Macromolecule #46: 30S ribosomal protein S13
+Macromolecule #47: 30S ribosomal protein S14
+Macromolecule #48: 30S ribosomal protein S15
+Macromolecule #49: 30S ribosomal protein S16
+Macromolecule #50: 30S ribosomal protein S17
+Macromolecule #51: 30S ribosomal protein S18
+Macromolecule #52: 30S ribosomal protein S19
+Macromolecule #53: 30S ribosomal protein S20
+Macromolecule #54: 30S ribosomal protein S21
+Macromolecule #55: Api137
+Macromolecule #58: Alternative stalled-ribosome rescue factor B
+Macromolecule #8: 23S ribosomal RNA
+Macromolecule #9: 5S ribosomal RNA
+Macromolecule #34: 16S ribosomal RNA
+Macromolecule #56: P-site tRNAPhe
+Macromolecule #57: mRNA
+Macromolecule #59: MAGNESIUM ION
+Macromolecule #60: ZINC ION
+Macromolecule #61: SODIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: 50 mM HEPES, 30 mM KCl, 7 mM MgCl2 |
|---|---|
| Grid | Model: Quantifoil R3.5/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Details: Custom-made glow-discharge instrument |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER / Details: Manual blotting & plunge-freezing. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: Cs image corrector (CEOS company) |
| Details | Aberration corrections performed using Cs image corrector (CEOS company) |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Average electron dose: 50.0 e/Å2 Details: Images were collected in movie mode at 40 fractions per image per second |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 0.0025 µm / Calibrated defocus min: 0.2 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm / Nominal defocus max: 0.0025 µm / Nominal defocus min: 0.2 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL | ||||||||||
| Output model |  PDB-6yss: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)