[English] 日本語
 Yorodumi
Yorodumi- EMDB-10797: Xenorhabdus nematophila XptA1 in complex with porcine mucosa heparin -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10797 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
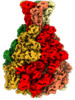
| Title | Xenorhabdus nematophila XptA1 in complex with porcine mucosa heparin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | toxin / complex / glycan | |||||||||
| Function / homology | TcA receptor binding domain / TcA receptor binding domain / Insecticidal toxin complex/plasmid virulence protein / Tc toxin complex TcA, C-terminal TcB-binding domain / Neuraminidase-like domain / Salmonella virulence plasmid 28.1kDa A protein / Tc toxin complex TcA C-terminal TcB-binding domain / Neuraminidase-like domain / A component of insecticidal toxin complex (Tc) Function and homology information Function and homology information | |||||||||
| Biological species |  Xenorhabdus nematophila (bacteria) Xenorhabdus nematophila (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Roderer D / Broecker F / Sitsel O / Kaplonek P / Leidreiter F / Seeberger PH / Raunser S | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Glycan-dependent cell adhesion mechanism of Tc toxins. Authors: Daniel Roderer / Felix Bröcker / Oleg Sitsel / Paulina Kaplonek / Franziska Leidreiter / Peter H Seeberger / Stefan Raunser /   Abstract: Toxin complex (Tc) toxins are virulence factors of pathogenic bacteria. Tcs are composed of three subunits: TcA, TcB and TcC. TcA facilitates receptor-toxin interaction and membrane permeation, TcB ...Toxin complex (Tc) toxins are virulence factors of pathogenic bacteria. Tcs are composed of three subunits: TcA, TcB and TcC. TcA facilitates receptor-toxin interaction and membrane permeation, TcB and TcC form a toxin-encapsulating cocoon. While the mechanisms of holotoxin assembly and pore formation have been described, little is known about receptor binding of TcAs. Here, we identify heparins/heparan sulfates and Lewis antigens as receptors for different TcAs from insect and human pathogens. Glycan array screening reveals that all tested TcAs bind negatively charged heparins. Cryo-EM structures of Morganella morganii TcdA4 and Xenorhabdus nematophila XptA1 reveal that heparins/heparan sulfates unexpectedly bind to different regions of the shell domain, including receptor-binding domains. In addition, Photorhabdus luminescens TcdA1 binds to Lewis antigens with micromolar affinity. Here, the glycan interacts with the receptor-binding domain D of the toxin. Our results suggest a glycan dependent association mechanism of Tc toxins on the host cell surface. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10797.map.gz emd_10797.map.gz | 9.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10797-v30.xml emd-10797-v30.xml emd-10797.xml emd-10797.xml | 14.2 KB 14.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10797.png emd_10797.png | 152.2 KB | ||
| Filedesc metadata |  emd-10797.cif.gz emd-10797.cif.gz | 7.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10797 http://ftp.pdbj.org/pub/emdb/structures/EMD-10797 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10797 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10797 | HTTPS FTP |
-Related structure data
| Related structure data |  6yeyMC  6yewC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10797.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10797.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.21 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Xenorhabdus nematophila XptA1 pentamer in complex with porcine mu...
| Entire | Name: Xenorhabdus nematophila XptA1 pentamer in complex with porcine mucosa heparin |
|---|---|
| Components |
|
-Supramolecule #1: Xenorhabdus nematophila XptA1 pentamer in complex with porcine mu...
| Supramolecule | Name: Xenorhabdus nematophila XptA1 pentamer in complex with porcine mucosa heparin type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Xenorhabdus nematophila (bacteria) Xenorhabdus nematophila (bacteria) |
| Molecular weight | Theoretical: 1.4 MDa |
-Macromolecule #1: A component of insecticidal toxin complex (Tc)
| Macromolecule | Name: A component of insecticidal toxin complex (Tc) / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Xenorhabdus nematophila (bacteria) Xenorhabdus nematophila (bacteria) |
| Molecular weight | Theoretical: 287.120781 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIKVNELLDK INRKRSGDTL LLTNISFMSF SEFRHRTSGT LTWRETDFLY QQAHQESKQN KLEELRILSR ANPQLANITN LNITPSTLN NSYNSWFYGR AHRFVKPGSI ASIFSPAAYL TELYREAKDF HPDNSQYHLN KRRPDIASLA LTQNNMDEEI S TLSLSNEL ...String: MIKVNELLDK INRKRSGDTL LLTNISFMSF SEFRHRTSGT LTWRETDFLY QQAHQESKQN KLEELRILSR ANPQLANITN LNITPSTLN NSYNSWFYGR AHRFVKPGSI ASIFSPAAYL TELYREAKDF HPDNSQYHLN KRRPDIASLA LTQNNMDEEI S TLSLSNEL LLHNIQTLEK TDYNGVMKML STYRQTGMTP YHLPYESARQ AILLQDKNLT AFSRNTDVAE LMDPTSLLAI KT DISPELY QILVEEITPE NSTELMKKNF GTDDVLIFKS YASLARYYDL SYDELSLFVN LSFGKKNTNQ QYKNEQLITL VND GNDTAT ARLIKRTRKD FYDSHLNYAE LIPIKENEYK YNFSVKKTEP DHLDFRLQNG DKEYIYQDKN FVPIANTHYS IPIK LTTEQ ITNGITLRLW RVKPNPSDAI NANAHFKMME FPGDIFLLKL NKAIRLYKAT GISPEDIWQV IESIYDDLTI DSNVL GKLF YVQYYMQHYN ISVSDALVLC HSDISQYSTK QQPSHFTMLF NTPLLNGQEF SADNTKLDLT PGESKNHFYL GIMKRA FRV NDTELYTLWK LANGGTNPEF MCSIENLSLL YRVRLLADIH HLTVNELSML LSVSPYVNTK IALFSDTALT QLISFLF QC TQWLTTQKWS VSDVFLMTTD NYSTVLTPDI ENLITTLSNG LSTLSLGDDE LIRAAAPLIA ASIQMDSAKT AETILLWI N QIKPQGLTFD DFMIIAANRD RSENETSNMV AFCQVLGQLS LIVRNIGLSE NELTLLVTKP EKFQSETTAL QHDLPTLQA LTRFHAVIMR CGSYATEILT ALELGALTAE QLAVALKFDA QVVTQALQQT DLGVNTFTNW RTIDVTLQWL DVAATLGITP DGVAALIKL KYVGEPETPM PTFDDWQAAS TLLQAGLNSQ QSDQLQAWLD EATTTAASAY YIKNGAPQQI KSRDELYSYL L IDNQVSAQ VKTTRVAEAI ASIQLYVNRA LNNVEGKVSK PVKTRQFFCD WETYNRRYST WAGVSELAYY PENYIDPTIR IG QTGMMNN LLQQLSQSQL NIDTVEDSFK NYLTAFEDVA NLQVISGYHD SINVNEGLTY LIGYSQTEPR IYYWRNVDHQ KCQ HGQFAA NAWGEWKKIE IPINVWQENI RPVIYKSRLY LLWLEQKELK NESEDGKIDI TDYILKLSHI RYDGSWSSPF NFNV TDKIE NLINKKASIG MYCSSDYEKD VIIVYFHEKK DNYSFNSLPA REGMTINPDM TLSILTENDL DAIVKSTLSE LDTRT EYKV NNQFATDYLA EYKESITTKN KLASFTGNIF DLSYISPGNG HINLTFNPSM EINFSKGNIY NDEVKYLLSM VEDETV ILF DYDRHDEMLG KEEEVFHYGT LDFIISIDLK NAEYFRVLMH LRTKEKIPRK SEIGVGINYD YESNDAEFKL DTNIVLD WK DNTGVWHTIC ESFTNDVSII NNMGNIAALF LREDPCVYLC SIATDIKIAS SMIEQIQDKN ISFLLKNGSD ILVELNAE D HVASKPSHES DPMVYDFNQV KVDIEGYDIP LVSEFIIKQP DGGYNDIVIE SPIHIKLKSK DTSNVISLHK MPSGTQYMQ IGPYRTRLNT LFSRKLAERA NIGIDNVLSM ETQNLPEPQL GEGFYATFKL PPYNKEEHGD ERWFKIHIGN IDGNSARQPY YEGMLSDIE TTVTLFVPYA KGYYIREGVR LGVGYKKIIY DKSWESAFFY FDETKNQFIF INDADHDSGM TQQGIVKNIK K YKGFIHVV VMKNNTEPMD FNGANAIYFW ELFYYTPMMV FQRLLQEQNF TESTRWLRYI WNPAGYSVQG EMQDYYWNVR PL EEDTSWN ANPLDSVDPD AVAQHDPMHY KVATFMKMLD LLITRGDSAY RQLERDTLNE AKMWYVQALT LLGDEPYFSL DND WSEPRL EEAASQTMRH HYQHKMLQLR QRAALPTKRT ANSLTALFLP QINKKLQGYW QTLTQRLYNL RHNLTIDGQP LSLS LYATP ADPSMLLSAA ITASQGGGDL PHAVMPMYRF PVILENAKWG VSQLIQFGNT LLSITERQDA EALAEILQTQ GSELA LQSI KMQDKVMAEI DADKLALQES RHGAQSRFDS FNTLYDEDVN AGEKQAMDLY LSSSVLSTSG TALHMAAAAA DLVPNI YGF AVGGSRFGAL FNASAIGIEI SASATRIAAD KISQSEIYRR RRQEWEIQRN NAEAEIKQID AQLATLAVRR EAAVLQK NY LETQQAQTQA QLAFLQSKFS NAALYNWLRG RLSAIYYQFY DLAVSLCLMA EQTYQYELNN AAAHFIKPGA WHGTYAGL L AGETLMLNLA QMEKSYLEKD ERALEVTRTV SLAEVYAGLT ENSFILKDKV TELVNAGEGS AGTTLNGLNV EGTQLQASL KLSDLNIATD YPDGLGNTRR IKQISVTLPA LLGPYQDVRA ILSYGGSTMM PRGCKAIAIS HGMNDSGQFQ MDFNDAKYLP FEGLPVADT GTLTLSFPGI SGKQKSLLLS LSDIILHIRY TIRS UniProtKB: A component of insecticidal toxin complex (Tc) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 285 K / Instrument: FEI VITROBOT MARK IV |
| Details | 0.10 mg/ml XptA1 were pre-applied on the grid for 30s. Subsequently, the grid was manually blotted and 0.30 mg/ml porcine mucosa heparin was applied and incubated for 4 min. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number real images: 1855 / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















