[English] 日本語
 Yorodumi
Yorodumi- EMDB-1069: Visualization of the domain structure of an L-type Ca2+ channel u... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1069 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. | |||||||||
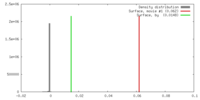
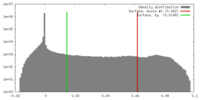
 Map data Map data | 3D reconstruction of the skeletal muscle dihydropyridin receptor (DHPR, L-type calcium channel) from single particle images. Low-pass filtered at 23A resolution. Ref.: J Mol Biol. 2003 Sep 5;332(1):171-82 If displayed with chimera (http://www.cgl.ucsf.edu/chimera/download) threshold level for published protein surface (white transparent) = 0.0626 threshold level for published core density (red) = 0.0763 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / negative staining / Resolution: 21.0 Å | |||||||||
 Authors Authors | Wolf M / Eberhart A / Glossmann H / Striessnig J / Grigorieff N | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2003 Journal: J Mol Biol / Year: 2003Title: Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. Authors: M Wolf / A Eberhart / H Glossmann / J Striessnig / N Grigorieff /  Abstract: The three-dimensional structure of the skeletal muscle voltage-gated L-type calcium channel (Ca(v)1.1; dihydropyridine receptor, DHPR) was determined using electron cryo-microscopy and single- ...The three-dimensional structure of the skeletal muscle voltage-gated L-type calcium channel (Ca(v)1.1; dihydropyridine receptor, DHPR) was determined using electron cryo-microscopy and single-particle averaging. The structure shows a single channel complex with an approximate total molecular mass of 550 kDa, corresponding to the five known subunits of the DHPR, and bound detergent and lipid. Features visible in our structure together with antibody labeling of the beta and alpha(2) subunits allowed us to assign locations for four of the five subunits within the structure. The most striking feature of the structure is the extra-cellular alpha(2) subunit that protrudes from the membrane domain in close proximity to the alpha(1) subunit. The cytosolic beta subunit is located close to the membrane and adjacent to subunits alpha(1), gamma and delta. Our structure correlates well with the functional and biochemical data available for this channel and suggests a three-dimensional model for the excitation-contraction coupling complex consisting of DHPR tetrads and the calcium release channel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1069.map.gz emd_1069.map.gz | 7.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1069-v30.xml emd-1069-v30.xml emd-1069.xml emd-1069.xml | 11.4 KB 11.4 KB | Display Display |  EMDB header EMDB header |
| Images |  1069.gif 1069.gif | 42.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1069 http://ftp.pdbj.org/pub/emdb/structures/EMD-1069 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1069 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1069 | HTTPS FTP |
-Validation report
| Summary document |  emd_1069_validation.pdf.gz emd_1069_validation.pdf.gz | 190.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1069_full_validation.pdf.gz emd_1069_full_validation.pdf.gz | 189.9 KB | Display | |
| Data in XML |  emd_1069_validation.xml.gz emd_1069_validation.xml.gz | 5.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1069 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1069 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1069 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1069 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1069.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1069.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of the skeletal muscle dihydropyridin receptor (DHPR, L-type calcium channel) from single particle images. Low-pass filtered at 23A resolution. Ref.: J Mol Biol. 2003 Sep 5;332(1):171-82 If displayed with chimera (http://www.cgl.ucsf.edu/chimera/download) threshold level for published protein surface (white transparent) = 0.0626 threshold level for published core density (red) = 0.0763 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.39 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : skeletal muscle dihydropyridine receptor
| Entire | Name: skeletal muscle dihydropyridine receptor |
|---|---|
| Components |
|
-Supramolecule #1000: skeletal muscle dihydropyridine receptor
| Supramolecule | Name: skeletal muscle dihydropyridine receptor / type: sample / ID: 1000 / Details: channel complex is solubilized in Digitonin Oligomeric state: monodisperse channel-detergent complex consisting of 5 subunits Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 800 KDa / Theoretical: 550 KDa / Method: gel filtration chromatography |
-Macromolecule #1: DHPR with subunits alpha1, alpha2, beta, gamma, delta
| Macromolecule | Name: DHPR with subunits alpha1, alpha2, beta, gamma, delta / type: protein_or_peptide / ID: 1 Name.synonym: DHPR, dihydropyridine receptor, CAv1.1, L type calcium channel Details: additional MW due to detergent and residual lipid; solubilized in Digitonin Number of copies: 1 Oligomeric state: monodisperse channel detergent complex with 5 subunits Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 550 KDa / Theoretical: 800 KDa |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 50mM Tris-HCl pH7.4, 150mM NaCl, 25uM CaCl2, 1mM Iodoacetamide, 0.1mM Benzamidine, 0.1mM PMSF, 0.1% (w/v) Digitonin. |
| Staining | Type: NEGATIVE Details: 5ul sample on holey carbon grids (Quantifoil) were vitrified by plunging into liqid ethane after blotting with filter paper. |
| Grid | Details: Quantifoil (R) holey carbon copper grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 20 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification instrument: custom plunger Method: Blot 5ul sample on negative glow-discharged grids for 5-10sec with Whatman filter paper before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 90 K / Max: 95 K / Average: 90 K |
| Alignment procedure | Legacy - Astigmatism: obj lens astigmatism was corrected at 270,000x magnification |
| Date | Feb 5, 2002 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 180 / Average electron dose: 9 e/Å2 Details: full scan resolution was downsampled by 3x3 pixel averaging. Od range: 1 / Bits/pixel: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 3.0 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo holder Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: per micrograph, CTFFIT3 |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 21.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: MRC, IMAGIC, FREALIGN Details: FREALIGN does not use class averages but performs an orientation search of each individual particle incl. CTF correction. The final map was reconstructed from contributions of 14,056 ...Details: FREALIGN does not use class averages but performs an orientation search of each individual particle incl. CTF correction. The final map was reconstructed from contributions of 14,056 particles and sharpened with a negative temperature factor of 500. The absolute handedness of the reconstruction was not determined. Number images used: 14056 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)