+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10539 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Processive human polymerase delta holoenzyme | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Protein / REPLICATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationdelta DNA polymerase complex / DNA synthesis involved in UV-damage excision repair / zeta DNA polymerase complex / nucleotide-excision repair complex / Cytosolic iron-sulfur cluster assembly / positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity ...delta DNA polymerase complex / DNA synthesis involved in UV-damage excision repair / zeta DNA polymerase complex / nucleotide-excision repair complex / Cytosolic iron-sulfur cluster assembly / positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity / nucleotide-excision repair, DNA gap filling / nuclear lamina / Polymerase switching / positive regulation of DNA-directed DNA polymerase activity / 3'-5'-DNA exonuclease activity / Processive synthesis on the lagging strand / DNA replication proofreading / MutLalpha complex binding / PCNA complex / Telomere C-strand (Lagging Strand) Synthesis / Removal of the Flap Intermediate / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / Transcription of E2F targets under negative control by DREAM complex / Polymerase switching on the C-strand of the telomere / replisome / Processive synthesis on the C-strand of the telomere / response to L-glutamate / Removal of the Flap Intermediate from the C-strand / aggresome / Hydrolases; Acting on ester bonds; Exodeoxyribonucleases producing 5'-phosphomonoesters / DNA biosynthetic process / error-free translesion synthesis / DNA strand elongation involved in DNA replication / response to dexamethasone / DNA synthesis involved in DNA repair / histone acetyltransferase binding / DNA polymerase processivity factor activity / leading strand elongation / G1/S-Specific Transcription / nuclear replication fork / replication fork processing / SUMOylation of DNA replication proteins / PCNA-Dependent Long Patch Base Excision Repair / error-prone translesion synthesis / fatty acid homeostasis / response to cadmium ion / estrous cycle / mismatch repair / translesion synthesis / response to UV / cyclin-dependent protein kinase holoenzyme complex / base-excision repair, gap-filling / positive regulation of endothelial cell proliferation / DNA polymerase binding / epithelial cell differentiation / liver regeneration / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / positive regulation of DNA replication / nuclear estrogen receptor binding / replication fork / Translesion synthesis by REV1 / positive regulation of DNA repair / Translesion synthesis by POLK / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / male germ cell nucleus / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / Recognition of DNA damage by PCNA-containing replication complex / receptor tyrosine kinase binding / DNA-templated DNA replication / HDR through Homologous Recombination (HRR) / cellular response to xenobiotic stimulus / Dual Incision in GG-NER / cellular response to hydrogen peroxide / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / cellular response to UV / response to estradiol / E3 ubiquitin ligases ubiquitinate target proteins / heart development / 4 iron, 4 sulfur cluster binding / chromatin organization / protein-macromolecule adaptor activity / DNA-directed DNA polymerase / damaged DNA binding / DNA-directed DNA polymerase activity / DNA replication / chromosome, telomeric region / nuclear body / nucleotide binding / DNA repair / chromatin binding / centrosome / chromatin / protein-containing complex binding / enzyme binding / negative regulation of transcription by RNA polymerase II / DNA binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
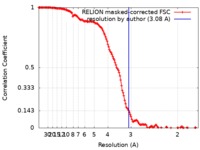
| Method | single particle reconstruction / cryo EM / Resolution: 3.08 Å | |||||||||
 Authors Authors | Lancey C / Hamdan SM | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structure of the processive human Pol δ holoenzyme. Authors: Claudia Lancey / Muhammad Tehseen / Vlad-Stefan Raducanu / Fahad Rashid / Nekane Merino / Timothy J Ragan / Christos G Savva / Manal S Zaher / Afnan Shirbini / Francisco J Blanco / Samir M ...Authors: Claudia Lancey / Muhammad Tehseen / Vlad-Stefan Raducanu / Fahad Rashid / Nekane Merino / Timothy J Ragan / Christos G Savva / Manal S Zaher / Afnan Shirbini / Francisco J Blanco / Samir M Hamdan / Alfredo De Biasio /    Abstract: In eukaryotes, DNA polymerase δ (Pol δ) bound to the proliferating cell nuclear antigen (PCNA) replicates the lagging strand and cooperates with flap endonuclease 1 (FEN1) to process the Okazaki ...In eukaryotes, DNA polymerase δ (Pol δ) bound to the proliferating cell nuclear antigen (PCNA) replicates the lagging strand and cooperates with flap endonuclease 1 (FEN1) to process the Okazaki fragments for their ligation. We present the high-resolution cryo-EM structure of the human processive Pol δ-DNA-PCNA complex in the absence and presence of FEN1. Pol δ is anchored to one of the three PCNA monomers through the C-terminal domain of the catalytic subunit. The catalytic core sits on top of PCNA in an open configuration while the regulatory subunits project laterally. This arrangement allows PCNA to thread and stabilize the DNA exiting the catalytic cleft and recruit FEN1 to one unoccupied monomer in a toolbelt fashion. Alternative holoenzyme conformations reveal important functional interactions that maintain PCNA orientation during synthesis. This work sheds light on the structural basis of Pol δ's activity in replicating the human genome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10539.map.gz emd_10539.map.gz | 15.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10539-v30.xml emd-10539-v30.xml emd-10539.xml emd-10539.xml | 30 KB 30 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10539_fsc.xml emd_10539_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_10539.png emd_10539.png | 58.4 KB | ||
| Filedesc metadata |  emd-10539.cif.gz emd-10539.cif.gz | 9.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10539 http://ftp.pdbj.org/pub/emdb/structures/EMD-10539 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10539 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10539 | HTTPS FTP |
-Related structure data
| Related structure data |  6tnyMC  6s1mC  6s1nC  6s1oC  6tnzC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10823 (Title: Cryo electron micrographs of Pol delta-PCNA-DNA-FEN1 sample EMPIAR-10823 (Title: Cryo electron micrographs of Pol delta-PCNA-DNA-FEN1 sampleData size: 1.6 TB Data #1: Cryo-electron micrographs of Pol delta-FEN1 toolbelt [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10539.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10539.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.87 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Toolbelt
+Supramolecule #1: Toolbelt
+Supramolecule #2: DNA polymerase
+Supramolecule #3: Proliferating cell nuclear antigen
+Supramolecule #4: DNA primer, template
+Macromolecule #1: DNA polymerase delta catalytic subunit
+Macromolecule #2: DNA polymerase delta subunit 2
+Macromolecule #3: DNA polymerase delta subunit 3
+Macromolecule #4: DNA polymerase delta subunit 4
+Macromolecule #5: Proliferating cell nuclear antigen
+Macromolecule #6: DNA primer
+Macromolecule #7: DNA template
+Macromolecule #8: ZINC ION
+Macromolecule #9: IRON/SULFUR CLUSTER
+Macromolecule #10: THYMIDINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 300 sec. | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||||||||
| Details | Complex separated by gel filtration |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 77.0 K |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number grids imaged: 1 / Number real images: 5071 / Average exposure time: 3.0 sec. / Average electron dose: 44.0 e/Å2 / Details: Data were collected in super resolution mode |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 57471 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.1 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)