+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10434 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ ER membrane bound ribosome-free translocon | |||||||||
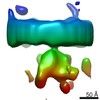
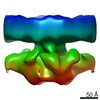
 Map data Map data | Putative ribosome-free translocon on ER membrane of intact P19 cells | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
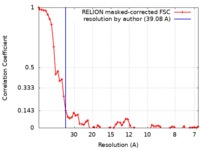
| Method | subtomogram averaging / cryo EM / Resolution: 39.08 Å | |||||||||
 Authors Authors | Martinez-Sanchez A / Lucic V / Chakraborty S | |||||||||
| Funding support |  Spain, 1 items Spain, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2020 Journal: Nat Methods / Year: 2020Title: Template-free detection and classification of membrane-bound complexes in cryo-electron tomograms. Authors: Antonio Martinez-Sanchez / Zdravko Kochovski / Ulrike Laugks / Johannes Meyer Zum Alten Borgloh / Saikat Chakraborty / Stefan Pfeffer / Wolfgang Baumeister / Vladan Lučić /  Abstract: With faithful sample preservation and direct imaging of fully hydrated biological material, cryo-electron tomography provides an accurate representation of molecular architecture of cells. However, ...With faithful sample preservation and direct imaging of fully hydrated biological material, cryo-electron tomography provides an accurate representation of molecular architecture of cells. However, detection and precise localization of macromolecular complexes within cellular environments is aggravated by the presence of many molecular species and molecular crowding. We developed a template-free image processing procedure for accurate tracing of complex networks of densities in cryo-electron tomograms, a comprehensive and automated detection of heterogeneous membrane-bound complexes and an unsupervised classification (PySeg). Applications to intact cells and isolated endoplasmic reticulum (ER) allowed us to detect and classify small protein complexes. This classification provided sufficiently homogeneous particle sets and initial references to allow subsequent de novo subtomogram averaging. Spatial distribution analysis showed that ER complexes have different localization patterns forming nanodomains. Therefore, this procedure allows a comprehensive detection and structural analysis of complexes in situ. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10434.map.gz emd_10434.map.gz | 1.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10434-v30.xml emd-10434-v30.xml emd-10434.xml emd-10434.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10434_fsc.xml emd_10434_fsc.xml | 5.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_10434.png emd_10434.png | 16 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10434 http://ftp.pdbj.org/pub/emdb/structures/EMD-10434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10434 | HTTPS FTP |
-Validation report
| Summary document |  emd_10434_validation.pdf.gz emd_10434_validation.pdf.gz | 221.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10434_full_validation.pdf.gz emd_10434_full_validation.pdf.gz | 220.8 KB | Display | |
| Data in XML |  emd_10434_validation.xml.gz emd_10434_validation.xml.gz | 9.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10434 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10434 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10434 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10434 | HTTPS FTP |
-Related structure data
| Related structure data |  0074C  0075C  0084C  0085C  0086C  0087C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10434.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10434.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Putative ribosome-free translocon on ER membrane of intact P19 cells | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.42 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Intact P19 cells
| Entire | Name: Intact P19 cells |
|---|---|
| Components |
|
-Supramolecule #1: Intact P19 cells
| Supramolecule | Name: Intact P19 cells / type: cell / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 90 % / Chamber temperature: 310.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 0.25 µm / Nominal defocus min: 0.2 µm / Nominal magnification: 42000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)