[English] 日本語
 Yorodumi
Yorodumi- EMDB-0936: Cryo-EM structure of the air-oxidized photosynthetic alternative ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0936 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the air-oxidized photosynthetic alternative complex III from Roseiflexus castenholzii | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | quinol:electron acceptor oxidoreductase / PHOTOSYNTHESIS | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmolybdopterin cofactor binding / electron transfer activity / oxidoreductase activity / heme binding / metal ion binding / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Roseiflexus castenholzii (strain DSM 13941 / HLO8) (bacteria) Roseiflexus castenholzii (strain DSM 13941 / HLO8) (bacteria) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||||||||
 Authors Authors | Shi Y / Xin YY | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2020 Journal: Sci Adv / Year: 2020Title: Cryo-EM structures of the air-oxidized and dithionite-reduced photosynthetic alternative complex III from . Authors: Yang Shi / Yueyong Xin / Chao Wang / Robert E Blankenship / Fei Sun / Xiaoling Xu /   Abstract: Alternative complex III (ACIII) is a multisubunit quinol:electron acceptor oxidoreductase that couples quinol oxidation with transmembrane proton translocation in both the respiratory and ...Alternative complex III (ACIII) is a multisubunit quinol:electron acceptor oxidoreductase that couples quinol oxidation with transmembrane proton translocation in both the respiratory and photosynthetic electron transport chains of bacteria. The coupling mechanism, however, is poorly understood. Here, we report the cryo-EM structures of air-oxidized and dithionite-reduced ACIII from the photosynthetic bacterium at 3.3- and 3.5-Å resolution, respectively. We identified a menaquinol binding pocket and an electron transfer wire comprising six hemes and four iron-sulfur clusters that is capable of transferring electrons to periplasmic acceptors. We detected a proton translocation passage in which three strictly conserved, mid-passage residues are likely essential for coupling the redox-driven proton translocation across the membrane. These results allow us to propose a previously unrecognized coupling mechanism that links the respiratory and photosynthetic functions of ACIII. This study provides a structural basis for further investigation of the energy transformation mechanisms in bacterial photosynthesis and respiration. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0936.map.gz emd_0936.map.gz | 26.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0936-v30.xml emd-0936-v30.xml emd-0936.xml emd-0936.xml | 22.4 KB 22.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0936.png emd_0936.png | 166.9 KB | ||
| Filedesc metadata |  emd-0936.cif.gz emd-0936.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0936 http://ftp.pdbj.org/pub/emdb/structures/EMD-0936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0936 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0936 | HTTPS FTP |
-Related structure data
| Related structure data |  6lodMC  0937C  6loeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0936.map.gz / Format: CCP4 / Size: 28.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0936.map.gz / Format: CCP4 / Size: 28.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
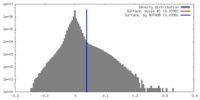
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Air-oxidized alternative complex III
+Supramolecule #1: Air-oxidized alternative complex III
+Macromolecule #1: MULTIHEME_CYTC domain-containing protein
+Macromolecule #2: Fe-S-cluster-containing hydrogenase components 1-like protein
+Macromolecule #3: Polysulphide reductase NrfD
+Macromolecule #4: Uncharacterized protein ActD
+Macromolecule #5: Cytochrome c domain-containing protein
+Macromolecule #6: Uncharacterized protein ActF
+Macromolecule #7: HEME C
+Macromolecule #8: [(2S)-2-octadecanoyloxypropyl] octadecanoate
+Macromolecule #9: IRON/SULFUR CLUSTER
+Macromolecule #10: FE3-S4 CLUSTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 59.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)