[English] 日本語
 Yorodumi
Yorodumi- EMDB-0688: Reconstitution and structure of a plant NLR resistosome conferrin... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0688 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Reconstitution and structure of a plant NLR resistosome conferring immunity | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | resistosome / PLANT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of defense response to bacterium / Tat protein binding / response to temperature stimulus / regulation of immune response / defense response / ADP binding / kinase activity / defense response to Gram-negative bacterium / cell surface receptor signaling pathway / non-specific serine/threonine protein kinase ...positive regulation of defense response to bacterium / Tat protein binding / response to temperature stimulus / regulation of immune response / defense response / ADP binding / kinase activity / defense response to Gram-negative bacterium / cell surface receptor signaling pathway / non-specific serine/threonine protein kinase / defense response to bacterium / protein serine kinase activity / protein serine/threonine kinase activity / ATP binding / nucleus / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Wang JZ / Wang J | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Reconstitution and structure of a plant NLR resistosome conferring immunity. Authors: Jizong Wang / Meijuan Hu / Jia Wang / Jinfeng Qi / Zhifu Han / Guoxun Wang / Yijun Qi / Hong-Wei Wang / Jian-Min Zhou / Jijie Chai /   Abstract: Nucleotide-binding, leucine-rich repeat receptors (NLRs) perceive pathogen effectors to trigger plant immunity. Biochemical mechanisms underlying plant NLR activation have until now remained poorly ...Nucleotide-binding, leucine-rich repeat receptors (NLRs) perceive pathogen effectors to trigger plant immunity. Biochemical mechanisms underlying plant NLR activation have until now remained poorly understood. We reconstituted an active complex containing the coiled-coil NLR ZAR1, the pseudokinase RKS1, uridylated protein kinase PBL2, and 2'-deoxyadenosine 5'-triphosphate (dATP), demonstrating the oligomerization of the complex during immune activation. The cryo-electron microscopy structure reveals a wheel-like pentameric ZAR1 resistosome. Besides the nucleotide-binding domain, the coiled-coil domain of ZAR1 also contributes to resistosome pentamerization by forming an α-helical barrel that interacts with the leucine-rich repeat and winged-helix domains. Structural remodeling and fold switching during activation release the very N-terminal amphipathic α helix of ZAR1 to form a funnel-shaped structure that is required for the plasma membrane association, cell death triggering, and disease resistance, offering clues to the biochemical function of a plant resistosome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0688.map.gz emd_0688.map.gz | 12.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0688-v30.xml emd-0688-v30.xml emd-0688.xml emd-0688.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0688.png emd_0688.png | 67.7 KB | ||
| Filedesc metadata |  emd-0688.cif.gz emd-0688.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0688 http://ftp.pdbj.org/pub/emdb/structures/EMD-0688 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0688 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0688 | HTTPS FTP |
-Related structure data
| Related structure data |  6j6iMC  0680C  6j5tC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0688.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0688.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.091 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : resistosome
| Entire | Name: resistosome |
|---|---|
| Components |
|
-Supramolecule #1: resistosome
| Supramolecule | Name: resistosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 130 KDa |
-Macromolecule #1: Probable serine/threonine-protein kinase PBL2
| Macromolecule | Name: Probable serine/threonine-protein kinase PBL2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 46.35643 KDa |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Sequence | String: MGNCLDSSAK VDNSNHSPHA NSASSGSKVS SKTSRSTGPS GLSTTSYSTD SSFGPLPTLR TEGEILSSPN LKAFTFNELK NATKNFRQD NLLGEGGFGC VFKGWIDQTS LTASRPGSGI VVAVKQLKPE GFQGHKEWLT EVNYLGQLSH PNLVLLVGYC A EGENRLLV ...String: MGNCLDSSAK VDNSNHSPHA NSASSGSKVS SKTSRSTGPS GLSTTSYSTD SSFGPLPTLR TEGEILSSPN LKAFTFNELK NATKNFRQD NLLGEGGFGC VFKGWIDQTS LTASRPGSGI VVAVKQLKPE GFQGHKEWLT EVNYLGQLSH PNLVLLVGYC A EGENRLLV YEFMPKGSLE NHLFRRGAQP LTWAIRMKVA VGAAKGLTFL HEAKSQVIYR DFKAANILLD ADFNAKLSDF GL AKAGPTG DNTHVSTKVI GTHGYAAPEY VATGRLTAKS DVYSFGVVLL ELISGRRAMD NSNGGNEYSL VDWATPYLGD KRK LFRIMD TKLGGQYPQK GAFTAANLAL QCLNPDAKLR PKMSEVLVTL EQLESVAKPG TKHTQMESPR FHHSSVMQKS PVRY SHDRP LLHMTPGASP LPSYTQSPRV R UniProtKB: Probable serine/threonine-protein kinase PBL2 |
-Macromolecule #2: Protein kinase superfamily protein
| Macromolecule | Name: Protein kinase superfamily protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 40.142375 KDa |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Sequence | String: MKKQYLKSGS GTRKEKDKAK RWFLDNGSIF LRELVADCNG KSIPIRSFSP EQILKATNNF DSSCFVSQDV YYKWYRGEIE DRSYMIKRF SEDEITGKRH RVKEVYNDIV LSARMSNHSN FLQLLGCCLE FPFPVLVFEF AEHGAMNQRG GVIVNGEESL L PWSVRLKI ...String: MKKQYLKSGS GTRKEKDKAK RWFLDNGSIF LRELVADCNG KSIPIRSFSP EQILKATNNF DSSCFVSQDV YYKWYRGEIE DRSYMIKRF SEDEITGKRH RVKEVYNDIV LSARMSNHSN FLQLLGCCLE FPFPVLVFEF AEHGAMNQRG GVIVNGEESL L PWSVRLKI GKEIANAVTY LHTAFPKIII HRDVKPMHVF LDKNWTAKLS DLSFSISLPE GKSRIEAEWV LGTFGYIDPL YH KTCFVTE YTDVYSFGIC LLVIITGKPA IMTISDGDLQ GILSLVRELC ENGKLDEVID PRLMKDITSG QRLQVEACVV LAL RCCKER DEDRPKMIQV AKELKQIEAS LKNSS UniProtKB: Serine/threonine-protein kinase ZRK1 |
-Macromolecule #3: Disease resistance RPP13-like protein 4
| Macromolecule | Name: Disease resistance RPP13-like protein 4 / type: protein_or_peptide / ID: 3 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 97.163977 KDa |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Sequence | String: MVDAVVTVFL EKTLNILEEK GRTVSDYRKQ LEDLQSELKY MQSFLKDAER QKRTNETLRT LVADLRELVY EAEDILVDCQ LADGDDGNE QRSSNAWLSR LHPARVPLQY KKSKRLQEIN ERITKIKSQV EPYFEFITPS NVGRDNGTDR WSSPVYDHTQ V VGLEGDKR ...String: MVDAVVTVFL EKTLNILEEK GRTVSDYRKQ LEDLQSELKY MQSFLKDAER QKRTNETLRT LVADLRELVY EAEDILVDCQ LADGDDGNE QRSSNAWLSR LHPARVPLQY KKSKRLQEIN ERITKIKSQV EPYFEFITPS NVGRDNGTDR WSSPVYDHTQ V VGLEGDKR KIKEWLFRSN DSQLLIMAFV GMGGLGKTTI AQEVFNDKEI EHRFERRIWV SVSQTFTEEQ IMRSILRNLG DA SVGDDIG TLLRKIQQYL LGKRYLIVMD DVWDKNLSWW DKIYQGLPRG QGGSVIVTTR SESVAKRVQA RDDKTHRPEL LSP DNSWLL FCNVAFAAND GTCERPELED VGKEIVTKCK GLPLTIKAVG GLLLCKDHVY HEWRRIAEHF QDELRGNTSE TDNV MSSLQ LSYDELPSHL KSCILTLSLY PEDCVIPKQQ LVHGWIGEGF VMWRNGRSAT ESGEDCFSGL TNRCLIEVVD KTYSG TIIT CKIHDMVRDL VIDIAKKDSF SNPEGLNCRH LGISGNFDEK QIKVNHKLRG VVSTTKTGEV NKLNSDLAKK FTDCKY LRV LDISKSIFDA PLSEILDEIA SLQHLACLSL SNTHPLIQFP RSMEDLHNLQ ILDASYCQNL KQLQPCIVLF KKLLVLD MT NCGSLECFPK GIGSLVKLEV LLGFKPARSN NGCKLSEVKN LTNLRKLGLS LTRGDQIEEE ELDSLINLSK LMSISINC Y DSYGDDLITK IDALTPPHQL HELSLQFYPG KSSPSWLSPH KLPMLRYMSI CSGNLVKMQE PFWGNENTHW RIEGLMLSS LSDLDMDWEV LQQSMPYLRT VTANWCPELE SFAIEDVGFR GGVWMKTPLH RT UniProtKB: Disease resistance RPP13-like protein 4 |
-Macromolecule #4: URIDINE-5'-MONOPHOSPHATE
| Macromolecule | Name: URIDINE-5'-MONOPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: U5P |
|---|---|
| Molecular weight | Theoretical: 324.181 Da |
| Chemical component information | 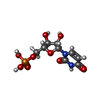 ChemComp-U: |
-Macromolecule #5: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE
| Macromolecule | Name: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 5 / Formula: DTP |
|---|---|
| Molecular weight | Theoretical: 491.182 Da |
| Chemical component information |  ChemComp-DTP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm / Nominal defocus max: 0.9 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















