[English] 日本語
 Yorodumi
Yorodumi- EMDB-0510: Protein Phosphatase 2A (Aalpha-B56alpha-Calpha) holoenzyme in com... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0510 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Protein Phosphatase 2A (Aalpha-B56alpha-Calpha) holoenzyme in complex with a Small Molecule Activator of PP2A (SMAP) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Holoenzyme complex / Phosphatase / Activator / HYDROLASE-ACTIVATOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationmeiotic spindle elongation / PP2A-mediated dephosphorylation of key metabolic factors / RNA polymerase II CTD heptapeptide repeat S2 phosphatase activity / RNA polymerase II CTD heptapeptide repeat S7 phosphatase activity / MASTL Facilitates Mitotic Progression / regulation of meiotic cell cycle process involved in oocyte maturation / protein phosphatase type 2A complex / mitotic sister chromatid separation / peptidyl-threonine dephosphorylation / meiotic sister chromatid cohesion, centromeric ...meiotic spindle elongation / PP2A-mediated dephosphorylation of key metabolic factors / RNA polymerase II CTD heptapeptide repeat S2 phosphatase activity / RNA polymerase II CTD heptapeptide repeat S7 phosphatase activity / MASTL Facilitates Mitotic Progression / regulation of meiotic cell cycle process involved in oocyte maturation / protein phosphatase type 2A complex / mitotic sister chromatid separation / peptidyl-threonine dephosphorylation / meiotic sister chromatid cohesion, centromeric / INTAC complex / RNA polymerase II CTD heptapeptide repeat S5 phosphatase activity / FAR/SIN/STRIPAK complex / Regulation of glycolysis by fructose 2,6-bisphosphate metabolism / Inhibition of replication initiation of damaged DNA by RB1/E2F1 / female meiotic nuclear division / protein phosphatase regulator activity / protein antigen binding / GABA receptor binding / APC truncation mutants have impaired AXIN binding / AXIN missense mutants destabilize the destruction complex / Truncations of AMER1 destabilize the destruction complex / ERKs are inactivated / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / Initiation of Nuclear Envelope (NE) Reformation / Beta-catenin phosphorylation cascade / Signaling by GSK3beta mutants / CTNNB1 S33 mutants aren't phosphorylated / CTNNB1 S37 mutants aren't phosphorylated / CTNNB1 S45 mutants aren't phosphorylated / CTNNB1 T41 mutants aren't phosphorylated / Co-stimulation by CD28 / RNA polymerase II transcription initiation surveillance / regulation of growth / M band / Disassembly of the destruction complex and recruitment of AXIN to the membrane / negative regulation of epithelial to mesenchymal transition / Co-inhibition by CTLA4 / Platelet sensitization by LDL / protein dephosphorylation / protein-serine/threonine phosphatase / negative regulation of glycolytic process through fructose-6-phosphate / ERK/MAPK targets / mesoderm development / protein serine/threonine phosphatase activity / vascular endothelial cell response to oscillatory fluid shear stress / positive regulation of NLRP3 inflammasome complex assembly / T cell homeostasis / regulation of cell differentiation / regulation of microtubule polymerization / regulation of G1/S transition of mitotic cell cycle / lateral plasma membrane / DARPP-32 events / chromosome, centromeric region / negative regulation of hippo signaling / negative regulation of protein localization to plasma membrane / Cyclin A/B1/B2 associated events during G2/M transition / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / spindle assembly / phosphoprotein phosphatase activity / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Recruitment of NuMA to mitotic centrosomes / Mitotic Prometaphase / Anchoring of the basal body to the plasma membrane / protein tyrosine phosphatase activity / EML4 and NUDC in mitotic spindle formation / negative regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / AURKA Activation by TPX2 / Resolution of Sister Chromatid Cohesion / meiotic cell cycle / chromosome segregation / RAF activation / Spry regulation of FGF signaling / negative regulation of canonical Wnt signaling pathway / RHO GTPases Activate Formins / PKR-mediated signaling / Degradation of beta-catenin by the destruction complex / response to lead ion / tau protein binding / kinase binding / Z disc / spindle pole / Negative regulation of MAPK pathway / Cyclin D associated events in G1 / Separation of Sister Chromatids / Regulation of TP53 Degradation / Regulation of PLK1 Activity at G2/M Transition / mitotic cell cycle / microtubule cytoskeleton / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / protein-containing complex assembly / neuron projection / intracellular signal transduction / membrane raft / protein heterodimerization activity / neuronal cell body Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
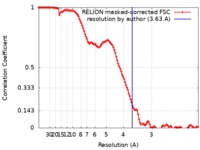
| Method | single particle reconstruction / cryo EM / Resolution: 3.63 Å | |||||||||
 Authors Authors | Huang W / Taylor D | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: Selective PP2A Enhancement through Biased Heterotrimer Stabilization. Authors: Daniel Leonard / Wei Huang / Sudeh Izadmehr / Caitlin M O'Connor / Danica D Wiredja / Zhizhi Wang / Nilesh Zaware / Yinghua Chen / Daniela M Schlatzer / Janna Kiselar / Nikhil Vasireddi / ...Authors: Daniel Leonard / Wei Huang / Sudeh Izadmehr / Caitlin M O'Connor / Danica D Wiredja / Zhizhi Wang / Nilesh Zaware / Yinghua Chen / Daniela M Schlatzer / Janna Kiselar / Nikhil Vasireddi / Stefan Schüchner / Abbey L Perl / Matthew D Galsky / Wenqing Xu / David L Brautigan / Egon Ogris / Derek J Taylor / Goutham Narla /   Abstract: Impairment of protein phosphatases, including the family of serine/threonine phosphatases designated PP2A, is essential for the pathogenesis of many diseases, including cancer. The ability of PP2A to ...Impairment of protein phosphatases, including the family of serine/threonine phosphatases designated PP2A, is essential for the pathogenesis of many diseases, including cancer. The ability of PP2A to dephosphorylate hundreds of proteins is regulated by over 40 specificity-determining regulatory "B" subunits that compete for assembly and activation of heterogeneous PP2A heterotrimers. Here, we reveal how a small molecule, DT-061, specifically stabilizes the B56α-PP2A holoenzyme in a fully assembled, active state to dephosphorylate selective substrates, such as its well-known oncogenic target, c-Myc. Our 3.6 Å structure identifies molecular interactions between DT-061 and all three PP2A subunits that prevent dissociation of the active enzyme and highlight inherent mechanisms of PP2A complex assembly. Thus, our findings provide fundamental insights into PP2A complex assembly and regulation, identify a unique interfacial stabilizing mode of action for therapeutic targeting, and aid in the development of phosphatase-based therapeutics tailored against disease specific phospho-protein targets. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0510.map.gz emd_0510.map.gz | 69.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0510-v30.xml emd-0510-v30.xml emd-0510.xml emd-0510.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0510_fsc.xml emd_0510_fsc.xml | 12 KB | Display |  FSC data file FSC data file |
| Images |  emd_0510.png emd_0510.png | 255.7 KB | ||
| Masks |  emd_0510_msk_1.map emd_0510_msk_1.map | 144.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-0510.cif.gz emd-0510.cif.gz | 7.7 KB | ||
| Others |  emd_0510_half_map_1.map.gz emd_0510_half_map_1.map.gz emd_0510_half_map_2.map.gz emd_0510_half_map_2.map.gz | 132.3 MB 134.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0510 http://ftp.pdbj.org/pub/emdb/structures/EMD-0510 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0510 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0510 | HTTPS FTP |
-Related structure data
| Related structure data |  6ntsMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0510.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0510.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.064 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_0510_msk_1.map emd_0510_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_0510_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_0510_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : PP2A Aalpha-B56alpha-Calpha holoenzyme in complex with a Small Mo...
| Entire | Name: PP2A Aalpha-B56alpha-Calpha holoenzyme in complex with a Small Molecule Activator of PP2A (SMAP) |
|---|---|
| Components |
|
-Supramolecule #1: PP2A Aalpha-B56alpha-Calpha holoenzyme in complex with a Small Mo...
| Supramolecule | Name: PP2A Aalpha-B56alpha-Calpha holoenzyme in complex with a Small Molecule Activator of PP2A (SMAP) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit...
| Macromolecule | Name: Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 65.378344 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAAADGDDSL YPIAVLIDEL RNEDVQLRLN SIKKLSTIAL ALGVERTRSE LLPFLTDTIY DEDEVLLALA EQLGTFTTLV GGPEYVHCL LPPLESLATV EETVVRDKAV ESLRAISHEH SPSDLEAHFV PLVKRLAGGD WFTSRTSACG LFSVCYPRVS S AVKAELRQ ...String: MAAADGDDSL YPIAVLIDEL RNEDVQLRLN SIKKLSTIAL ALGVERTRSE LLPFLTDTIY DEDEVLLALA EQLGTFTTLV GGPEYVHCL LPPLESLATV EETVVRDKAV ESLRAISHEH SPSDLEAHFV PLVKRLAGGD WFTSRTSACG LFSVCYPRVS S AVKAELRQ YFRNLCSDDT PMVRRAAASK LGEFAKVLEL DNVKSEIIPM FSNLASDEQD SVRLLAVEAC VNIAQLLPQE DL EALVMPT LRQAAEDKSW RVRYMVADKF TELQKAVGPE ITKTDLVPAF QNLMKDCEAE VRAAASHKVK EFCENLSADC REN VIMSQI LPCIKELVSD ANQHVKSALA SVIMGLSPIL GKDNTIEHLL PLFLAQLKDE CPEVRLNIIS NLDCVNEVIG IRQL SQSLL PAIVELAEDA KWRVRLAIIE YMPLLAGQLG VEFFDEKLNS LCMAWLVDHV YAIREAATSN LKKLVEKFGK EWAHA TIIP KVLAMSGDPN YLHRMTTLFC INVLSEVCGQ DITTKHMLPT VLRMAGDPVA NVRFNVAKSL QKIGPILDNS TLQSEV KPI LEKLTQDQDV DVKYFAQEAL TVLSLA UniProtKB: Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform |
-Macromolecule #2: Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit...
| Macromolecule | Name: Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit alpha isoform type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.266555 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSSSPPAGA ASAAISASEK VDGFTRKSVR KAQRQKRSQG SSQFRSQGSQ AELHPLPQLK DATSNEQQEL FCQKLQQCCI LFDFMDSVS DLKSKEIKRA TLNELVEYVS TNRGVIVESA YSDIVKMISA NIFRTLPPSD NPDFDPEEDE PTLEASWPHI Q LVYEFFLR ...String: MSSSSPPAGA ASAAISASEK VDGFTRKSVR KAQRQKRSQG SSQFRSQGSQ AELHPLPQLK DATSNEQQEL FCQKLQQCCI LFDFMDSVS DLKSKEIKRA TLNELVEYVS TNRGVIVESA YSDIVKMISA NIFRTLPPSD NPDFDPEEDE PTLEASWPHI Q LVYEFFLR FLESPDFQPS IAKRYIDQKF VQQLLELFDS EDPRERDFLK TVLHRIYGKF LGLRAFIRKQ INNIFLRFIY ET EHFNGVA ELLEILGSII NGFALPLKAE HKQFLMKVLI PMHTAKGLAL FHAQLAYCVV QFLEKDTTLT EPVIRGLLKF WPK TCSQKE VMFLGEIEEI LDVIEPTQFK KIEEPLFKQI SKCVSSSHFQ VAERALYFWN NEYILSLIEE NIDKILPIMF ASLY KISKE HWNPTIVALV YNVLKTLMEM NGKLFDDLTS SYKAERQREK KKELEREELW KKLEELKLKK ALEKQNSAYN MHSIL SNTS AE UniProtKB: Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit alpha isoform |
-Macromolecule #3: Serine/threonine-protein phosphatase 2A catalytic subunit alpha i...
| Macromolecule | Name: Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: protein-serine/threonine phosphatase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 35.0215 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDEKVFTKEL DQWIEQLNEC KQLSESQVKS LCEKAKEILT KESNVQEVRC PVTVCGDVHG QFHDLMELFR IGGKSPDTNY LFMGDYVNR GYYSVETVTL LVALKVRYRE RITILRGNHE SRQITQVYGF YDECLRKYGN ANVWKYFTDL FDYLPLTALV D GQIFCLHG ...String: MDEKVFTKEL DQWIEQLNEC KQLSESQVKS LCEKAKEILT KESNVQEVRC PVTVCGDVHG QFHDLMELFR IGGKSPDTNY LFMGDYVNR GYYSVETVTL LVALKVRYRE RITILRGNHE SRQITQVYGF YDECLRKYGN ANVWKYFTDL FDYLPLTALV D GQIFCLHG GLSPSIDTLD HIRALDRLQE VPHEGPMCDL LWSDPDDRGG WGISPRGAGY TFGQDISETF NHANGLTLVS RA HQLVMEG YNWCHDRNVV TIFSAPNYCY RCGNQAAIME LDDTLKYSFL QFDPAPRRGE PHVTYF(MLL) UniProtKB: Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform |
-Macromolecule #4: N-[(1R,2R,3S)-2-hydroxy-3-(10H-phenoxazin-10-yl)cyclohexyl]-4-(tr...
| Macromolecule | Name: N-[(1R,2R,3S)-2-hydroxy-3-(10H-phenoxazin-10-yl)cyclohexyl]-4-(trifluoromethoxy)benzene-1-sulfonamide type: ligand / ID: 4 / Number of copies: 1 / Formula: L2J |
|---|---|
| Molecular weight | Theoretical: 520.521 Da |
| Chemical component information | 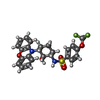 ChemComp-L2J: |
-Macromolecule #5: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.02 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)