[English] 日本語
 Yorodumi
Yorodumi- EMDB-0479: Cryo-EM structure of human TPC2 channel in the ligand-bound close... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0479 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human TPC2 channel in the ligand-bound closed state | |||||||||||||||
 Map data Map data | Human TPC2 channel in the ligand-bound closed state | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | channel / lysosome / TRANSPORT PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationendosome to lysosome transport of low-density lipoprotein particle / negative regulation of developmental pigmentation / intracellular pH reduction / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / NAADP-sensitive calcium-release channel activity / regulation of exocytosis / melanosome membrane / endolysosome membrane / phosphatidylinositol-3,5-bisphosphate binding / response to vitamin D ...endosome to lysosome transport of low-density lipoprotein particle / negative regulation of developmental pigmentation / intracellular pH reduction / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / NAADP-sensitive calcium-release channel activity / regulation of exocytosis / melanosome membrane / endolysosome membrane / phosphatidylinositol-3,5-bisphosphate binding / response to vitamin D / lysosome organization / ligand-gated sodium channel activity / monoatomic ion channel complex / smooth muscle contraction / voltage-gated calcium channel activity / receptor-mediated endocytosis of virus by host cell / release of sequestered calcium ion into cytosol / sodium ion transmembrane transport / calcium-mediated signaling / regulation of autophagy / Stimuli-sensing channels / calcium channel activity / intracellular calcium ion homeostasis / late endosome membrane / monoatomic ion transmembrane transport / lysosome / endosome membrane / endocytosis involved in viral entry into host cell / lysosomal membrane / protein kinase binding / identical protein binding / cytosol Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||||||||
 Authors Authors | She J / Zeng W | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Structural mechanisms of phospholipid activation of the human TPC2 channel. Authors: Ji She / Weizhong Zeng / Jiangtao Guo / Qingfeng Chen / Xiao-Chen Bai / Youxing Jiang /   Abstract: Mammalian two-pore channels (TPCs) regulate the physiological functions of the endolysosome. Here we present cryo-EM structures of human TPC2 (HsTPC2), a phosphatidylinositol 3,5-bisphosphate (PI(3,5) ...Mammalian two-pore channels (TPCs) regulate the physiological functions of the endolysosome. Here we present cryo-EM structures of human TPC2 (HsTPC2), a phosphatidylinositol 3,5-bisphosphate (PI(3,5)P)-activated, Na selective channel, in the ligand-bound and apo states. The apo structure captures the closed conformation, while the ligand-bound form features the channel in both open and closed conformations. Combined with functional analysis, these structures provide insights into the mechanism of PI(3,5)P-regulated gating of TPC2, which is distinct from that of TPC1. Specifically, the endolysosome-specific PI(3,5)P binds at the first 6-TM and activates the channel - independently of the membrane potential - by inducing a structural change at the pore-lining inner helix (IS6), which forms a continuous helix in the open state but breaks into two segments at Gly317 in the closed state. Additionally, structural comparison to the voltage-dependent TPC1 structure allowed us to identify Ile551 as being responsible for the loss of voltage dependence in TPC2. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0479.map.gz emd_0479.map.gz | 49 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0479-v30.xml emd-0479-v30.xml emd-0479.xml emd-0479.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0479.png emd_0479.png | 177.1 KB | ||
| Filedesc metadata |  emd-0479.cif.gz emd-0479.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0479 http://ftp.pdbj.org/pub/emdb/structures/EMD-0479 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0479 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0479 | HTTPS FTP |
-Related structure data
| Related structure data |  6nq2MC  0477C  0478C  6nq0C  6nq1C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0479.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0479.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human TPC2 channel in the ligand-bound closed state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : The complex of two-pore channel 2 with PI(3,5)P2
| Entire | Name: The complex of two-pore channel 2 with PI(3,5)P2 |
|---|---|
| Components |
|
-Supramolecule #1: The complex of two-pore channel 2 with PI(3,5)P2
| Supramolecule | Name: The complex of two-pore channel 2 with PI(3,5)P2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Two pore calcium channel protein 2
| Macromolecule | Name: Two pore calcium channel protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 85.671828 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSLEMAEPQA ESEPAAGGAR GGGGDWPAGL TTYRSIQVGP GAAARWDLCI DQAVVFIEDA IQYRSINHRV DASSMWLYRR YYSNVCQRT LSFTIFLILF LAFIETPSSL TSTADVRYRA APWEPPCGLT ESVEVLCLLV FAADLSVKGY LFGWAHFQKN L WLLGYLVV ...String: GSLEMAEPQA ESEPAAGGAR GGGGDWPAGL TTYRSIQVGP GAAARWDLCI DQAVVFIEDA IQYRSINHRV DASSMWLYRR YYSNVCQRT LSFTIFLILF LAFIETPSSL TSTADVRYRA APWEPPCGLT ESVEVLCLLV FAADLSVKGY LFGWAHFQKN L WLLGYLVV LVVSLVDWTV SLSLVCHEPL RIRRLLRPFF LLQNSSMMKK TLKCIRWSLP EMASVGLLLA IHLCLFTMFG ML LFAGGKQ DDGQDRERLT YFQNLPESLT SLLVLLTTAN NPDVMIPAYS KNRAYAIFFI VFTVIGSLFL MNLLTAIIYS QFR GYLMKS LQTSLFRRRL GTRAAFEVLS SMVGEGGAFP QAVGVKPQNL LQVLQKVQLD SSHKQAMMEK VRSYGSVLLS AEEF QKLFN ELDRSVVKEH PPRPEYQSPF LQSAQFLFGH YYFDYLGNLI ALANLVSICV FLVLDADVLP AERDDFILGI LNCVF IVYY LLEMLLKVFA LGLRGYLSYP SNVFDGLLTV VLLVLEISTL AVYRLPHPGW RPEMVGLLSL WDMTRMLNML IVFRFL RII PSMKPMAVVA STVLGLVQNM RAFGGILVVV YYVFAIIGIN LFRGVIVALP GNSSLAPANG SAPCGSFEQL EYWANNF DD FAAALVTLWN LMVVNNWQVF LDAYRRYSGP WSKIYFVLWW LVSSVIWVNL FLALILENFL HKWDPRSHLQ PLAGTPEA T YQMTVELLFR DILEEPEEDE LTERLSQHPH LWLCR UniProtKB: Two pore channel protein 2 |
-Macromolecule #2: (2R)-3-{[(S)-hydroxy{[(1S,2R,3R,4S,5S,6R)-2,4,6-trihydroxy-3,5-bi...
| Macromolecule | Name: (2R)-3-{[(S)-hydroxy{[(1S,2R,3R,4S,5S,6R)-2,4,6-trihydroxy-3,5-bis(phosphonooxy)cyclohexyl]oxy}phosphoryl]oxy}propane-1,2-diyl dioctanoate type: ligand / ID: 2 / Number of copies: 2 / Formula: EUJ |
|---|---|
| Molecular weight | Theoretical: 746.566 Da |
| Chemical component information | 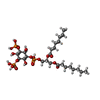 ChemComp-EUJ: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















