[English] 日本語
 Yorodumi
Yorodumi- EMDB-0041: Structure of paused transcription complex Pol II-DSIF-NELF, (Map 4) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of paused transcription complex Pol II-DSIF-NELF, (Map 4) | |||||||||
 Map data Map data | Globally refined upstream DNA, NGN, KOW1 selected particles (Map 4). | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
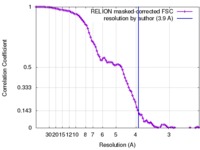
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Vos SM / Farnung L / Urlaub H / Cramer P | |||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structure of paused transcription complex Pol II-DSIF-NELF. Authors: Seychelle M Vos / Lucas Farnung / Henning Urlaub / Patrick Cramer /  Abstract: Metazoan gene regulation often involves the pausing of RNA polymerase II (Pol II) in the promoter-proximal region. Paused Pol II is stabilized by the protein complexes DRB sensitivity-inducing factor ...Metazoan gene regulation often involves the pausing of RNA polymerase II (Pol II) in the promoter-proximal region. Paused Pol II is stabilized by the protein complexes DRB sensitivity-inducing factor (DSIF) and negative elongation factor (NELF). Here we report the cryo-electron microscopy structure of a paused transcription elongation complex containing Sus scrofa Pol II and Homo sapiens DSIF and NELF at 3.2 Å resolution. The structure reveals a tilted DNA-RNA hybrid that impairs binding of the nucleoside triphosphate substrate. NELF binds the polymerase funnel, bridges two mobile polymerase modules, and contacts the trigger loop, thereby restraining Pol II mobility that is required for pause release. NELF prevents binding of the anti-pausing transcription elongation factor IIS (TFIIS). Additionally, NELF possesses two flexible 'tentacles' that can contact DSIF and exiting RNA. These results define the paused state of Pol II and provide the molecular basis for understanding the function of NELF during promoter-proximal gene regulation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0041.map.gz emd_0041.map.gz | 49.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0041-v30.xml emd-0041-v30.xml emd-0041.xml emd-0041.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0041_fsc.xml emd_0041_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_0041.png emd_0041.png | 180.8 KB | ||
| Masks |  emd_0041_msk_1.map emd_0041_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_0041_additional.map.gz emd_0041_additional.map.gz emd_0041_half_map_1.map.gz emd_0041_half_map_1.map.gz emd_0041_half_map_2.map.gz emd_0041_half_map_2.map.gz | 60 MB 49.7 MB 49.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0041 http://ftp.pdbj.org/pub/emdb/structures/EMD-0041 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0041 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0041 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0041.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0041.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Globally refined upstream DNA, NGN, KOW1 selected particles (Map 4). | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2277 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_0041_msk_1.map emd_0041_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed/B-factor sharpened upstream DNA, NGN, KOW1 selected particles...
| File | emd_0041_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed/B-factor sharpened upstream DNA, NGN, KOW1 selected particles (Map 4). Applied B-factor of -71. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 for globally refined upstream DNA,...
| File | emd_0041_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 for globally refined upstream DNA, NGN, KOW1 selected particles (Map 4). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 for globally refined upstream DNA,...
| File | emd_0041_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 for globally refined upstream DNA, NGN, KOW1 selected particles (Map 4). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : RNA Polymerase II-DSIF-PAF-SPT6 elongation complex (EC*)
| Entire | Name: RNA Polymerase II-DSIF-PAF-SPT6 elongation complex (EC*) |
|---|---|
| Components |
|
-Supramolecule #1: RNA Polymerase II-DSIF-PAF-SPT6 elongation complex (EC*)
| Supramolecule | Name: RNA Polymerase II-DSIF-PAF-SPT6 elongation complex (EC*) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#21, #23 |
|---|---|
| Molecular weight | Theoretical: 927.1 KDa |
-Supramolecule #2: RNA Polymerase II
| Supramolecule | Name: RNA Polymerase II / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#9, #11, #10, #12 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: associated proteins
| Supramolecule | Name: associated proteins / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #13, #16, #18, #23, #19-#22 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
-Supramolecule #4: Nucleic acids
| Supramolecule | Name: Nucleic acids / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #14-#15, #17 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Recombinant expression | Organism: synthetic construct (others) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Number real images: 11740 / Average exposure time: 9.0 sec. / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)