+ Open data
Open data
- Basic information
Basic information
| Entry |  |
|---|---|
 Sample Sample | Nucleolysin TIA-1 isoform p40 in complex with U15 RNA
|
| Function / homology | Isoform Short of Cytotoxic granule associated RNA binding protein TIA1 Function and homology information Function and homology information |
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
 Citation Citation |  Journal: Angew Chem Int Ed Engl / Year: 2017 Journal: Angew Chem Int Ed Engl / Year: 2017Title: Segmental, Domain-Selective Perdeuteration and Small-Angle Neutron Scattering for Structural Analysis of Multi-Domain Proteins. Authors: Miriam Sonntag / Pravin Kumar Ankush Jagtap / Bernd Simon / Marie-Sousai Appavou / Arie Geerlof / Ralf Stehle / Frank Gabel / Janosch Hennig / Michael Sattler /   Abstract: Multi-domain proteins play critical roles in fine-tuning essential processes in cellular signaling and gene regulation. Typically, multiple globular domains that are connected by flexible linkers ...Multi-domain proteins play critical roles in fine-tuning essential processes in cellular signaling and gene regulation. Typically, multiple globular domains that are connected by flexible linkers undergo dynamic rearrangements upon binding to protein, DNA or RNA ligands. RNA binding proteins (RBPs) represent an important class of multi-domain proteins, which regulate gene expression by recognizing linear or structured RNA sequence motifs. Here, we employ segmental perdeuteration of the three RNA recognition motif (RRM) domains in the RBP TIA-1 using Sortase A mediated protein ligation. We show that domain-selective perdeuteration combined with contrast-matched small-angle neutron scattering (SANS), SAXS and computational modeling provides valuable information to precisely define relative domain arrangements. The approach is generally applicable to study conformational arrangements of individual domains in multi-domain proteins and changes induced by ligand binding. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-Models
- Sample
Sample
 Sample Sample | Name: Nucleolysin TIA-1 isoform p40 in complex with U15 RNA / Entity id: 659 / 660 |
|---|---|
| Buffer | Name: 10 mM Potassium Phosphate 50 mM NaCl 10 mM DTT / pH: 6 |
| Entity #659 | Name: TIA-1 wild type / Type: protein / Description: Nucleolysin TIA-1 isoform p40 / Formula weight: 30.457 / Num. of mol.: 1 / Source: Homo sapiens / References: UniProt: P31483-2 Sequence: MEDEMPKTLY VGNLSRDVTE ALILQLFSQI GPCKNCKMIM DTAGNDPYCF VEFHEHRHAA AALAAMNGRK IMGKEVKVNW ATTPSSQKKD TSNHFHVFVG DLSPEITTED IKAAFAPFGR ISDARVVKDM ATGKSKGYGF VSFFNKWDAE NAIQQMGGQW LGGRQIRTNW ...Sequence: MEDEMPKTLY VGNLSRDVTE ALILQLFSQI GPCKNCKMIM DTAGNDPYCF VEFHEHRHAA AALAAMNGRK IMGKEVKVNW ATTPSSQKKD TSNHFHVFVG DLSPEITTED IKAAFAPFGR ISDARVVKDM ATGKSKGYGF VSFFNKWDAE NAIQQMGGQW LGGRQIRTNW ATRKPPAPKS TYESNTKQLS YDEVVNQSSP SNCTVYCGGV TSGLTEQLMR QTFSPFGQIM EIRVFPDKGY SFVRFNSHES AAHAIVSVNG TTIEGHVVKC YWGK |
| Entity #660 | Name: U15 / Type: RNA / Description: poly U 15mer / Formula weight: 4.611 / Num. of mol.: 1 Sequence: UUUUUUUUUU UUUUU |
-Experimental information
| Beam | Instrument name: Rigaku BioSAXS-1000 / City: Munich / 国: Germany  / Type of source: X-ray in house / Type of source: X-ray in house | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 100K / Pixsize x: 172 mm | ||||||||||||||||||||||||||||||||||||
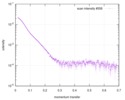
| Scan | Measurement date: Apr 28, 2016 / Unit: 1/A /
| ||||||||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||||||||||||||||||||
| Result |
|
 Movie
Movie Controller
Controller



 SASDCC3
SASDCC3